When Ice Melts To Form Liquid Energy Is
When Ice Melts To Form Liquid Energy Is - As ice melts into water, kinetic energy is being added to the. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. What happens to the kinetic energy of its molecules as ice melts into water? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and.
First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. As ice melts into water, kinetic energy is being added to the. What happens to the kinetic energy of its molecules as ice melts into water? In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and.
What happens to the kinetic energy of its molecules as ice melts into water? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. As ice melts into water, kinetic energy is being added to the. Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to.
What Is Latent Heat? » ScienceABC
Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. As ice melts into water, kinetic energy is being added to the. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and..
Ice cube melting Stock Photo Ice cube melting, Ice cube, Ice photography
In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. What happens to the kinetic energy of its molecules as ice melts into water? As ice melts into water,.
What Melts Ice the Fastest? Exploring Different Methods in a Science
In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. Melting of ice occurs in two steps: As ice melts into water, kinetic energy is being added to the..
Ice Cube Melting Science Project
Melting of ice occurs in two steps: First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. What happens to the kinetic energy of its molecules as ice melts into water? As ice melts into water, kinetic energy is being added to the. During the melting process, the kinetic energy provided through.
Melting to Keep Cool NOVA PBS
As ice melts into water, kinetic energy is being added to the. What happens to the kinetic energy of its molecules as ice melts into water? First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. During the melting process, the kinetic energy provided through heat enables the particles of ice to.
How Does Salt Melt Snow and Ice? LDP Watersheds
During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. What happens to the kinetic energy of its molecules as ice melts into water? In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. First the phase change occurs.
Ice Cubes Melting Process Sciencing
In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. As ice melts into water, kinetic energy is being added to the. During the melting process, the kinetic energy provided through heat.
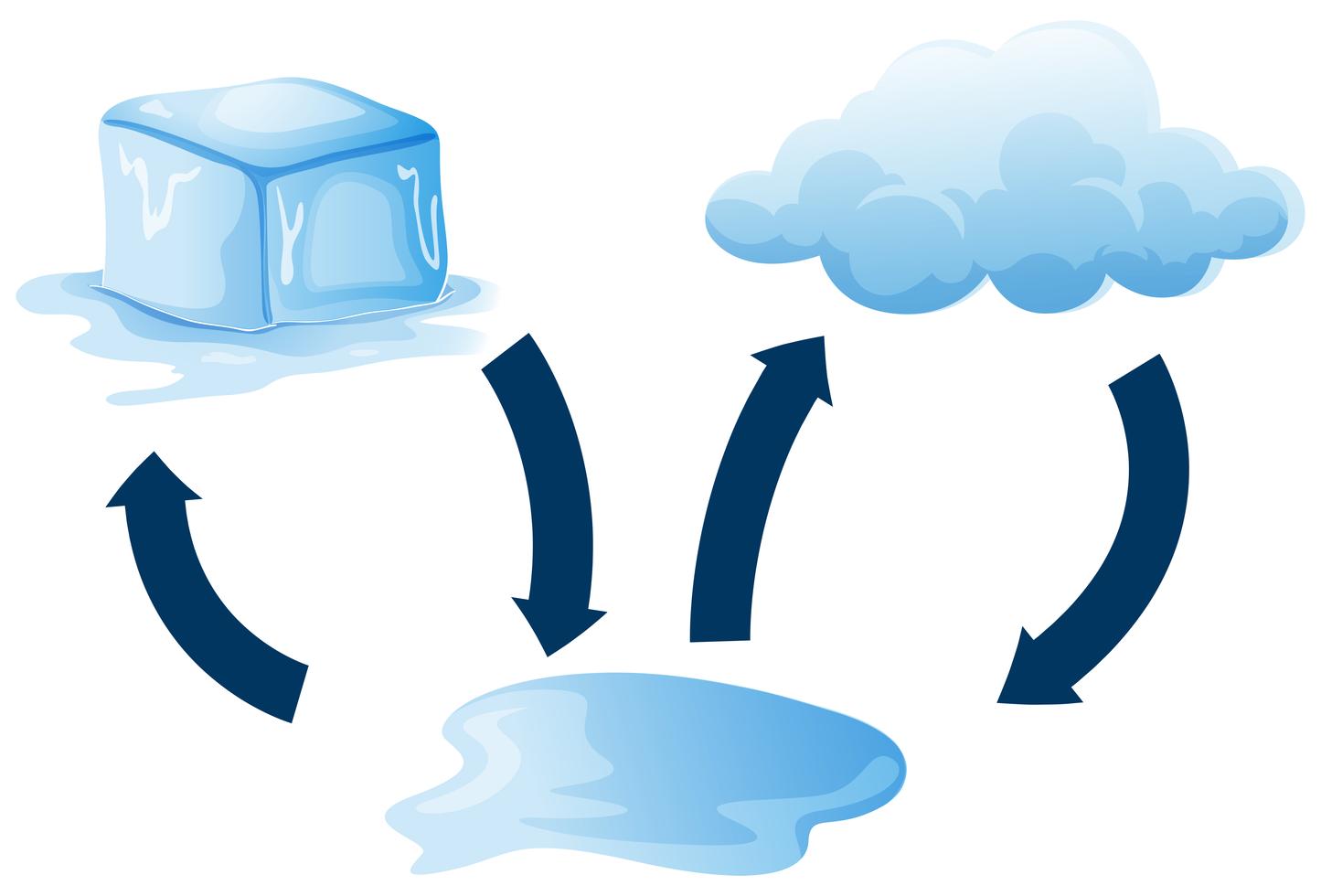
Diagram showing how ice melts 433823 Vector Art at Vecteezy
As ice melts into water, kinetic energy is being added to the. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. Melting of ice occurs in two steps: During the melting.
The Ice Is Melting Learn Something New
First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. As ice melts into water, kinetic energy is being added to the. Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. What happens to.
Why does ice cream melt? The science behind the scoop (and how to stop
First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through heat enables the particles of ice.
Melting Of Ice Occurs In Two Steps:
During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. What happens to the kinetic energy of its molecules as ice melts into water? In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to.