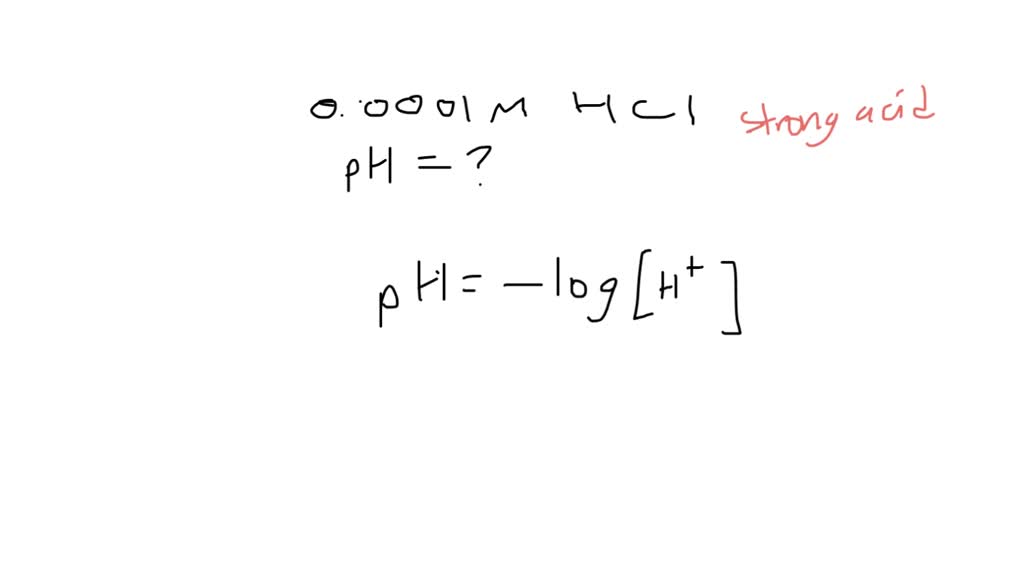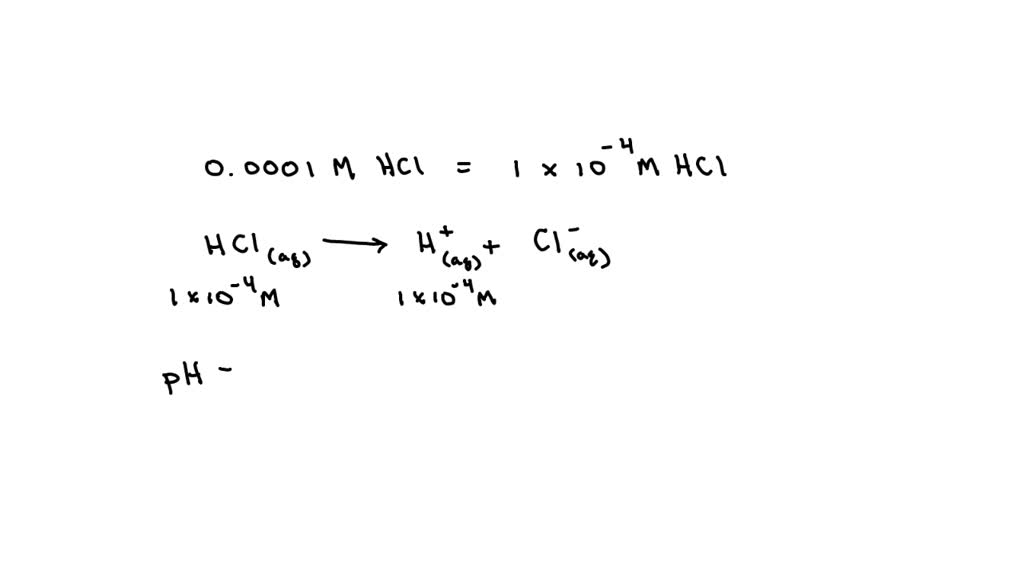What Is The Ph Of A 0 0001 M Hcl Solution
What Is The Ph Of A 0 0001 M Hcl Solution - Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. How ph value is increased when hcl acid solution is diluted. What is the ph of a 0.0001m hcl solution? The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! A lab assistant prepared a solution by adding a. Our expert help has broken down your. What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? What is the ph of 0.0001m hcl solution? The ph of 0.0001 n solution of koh will be:
How ph value is increased when hcl acid solution is diluted. A lab assistant prepared a solution by adding a. What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? What is the ph of a 0.0001m hcl solution? Our expert help has broken down your. Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. Aqueous solution of hcl has the ph = 4. The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of 0.0001m hcl solution?
The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of a 0.0001m hcl solution? A lab assistant prepared a solution by adding a. How ph value is increased when hcl acid solution is diluted. Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. The ph of 0.0001 n solution of koh will be: (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of 0.0001m hcl solution? What is the ph of a soution containing.0001 m hcl? What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed?
Solved What is the pH of a solution that is 0.0001 M HCl?
A lab assistant prepared a solution by adding a. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! How ph value is increased when hcl acid solution is diluted. The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of.
1 M TrisHCl (pH 7.4), (ML01774) Welgene
What is the ph of a 0.0001m hcl solution? A lab assistant prepared a solution by adding a. What is the ph of 0.0001m hcl solution? What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that.
The pH value of 10 M solution of HCl is
A lab assistant prepared a solution by adding a. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. What is the ph of a soution containing.0001 m hcl? What is the.
SOLVED Calculate the pH of a 0.0001 M HCl solution (Express your
Our expert help has broken down your. How ph value is increased when hcl acid solution is diluted. The ph of 0.0001 n solution of koh will be: The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it.
Find the pH of a 0.025 HCl (Hydrochloric acid) Solution YouTube
(remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? What is the ph of a 0.0001m hcl solution? Aqueous solution of hcl has the ph = 4. How ph value is.
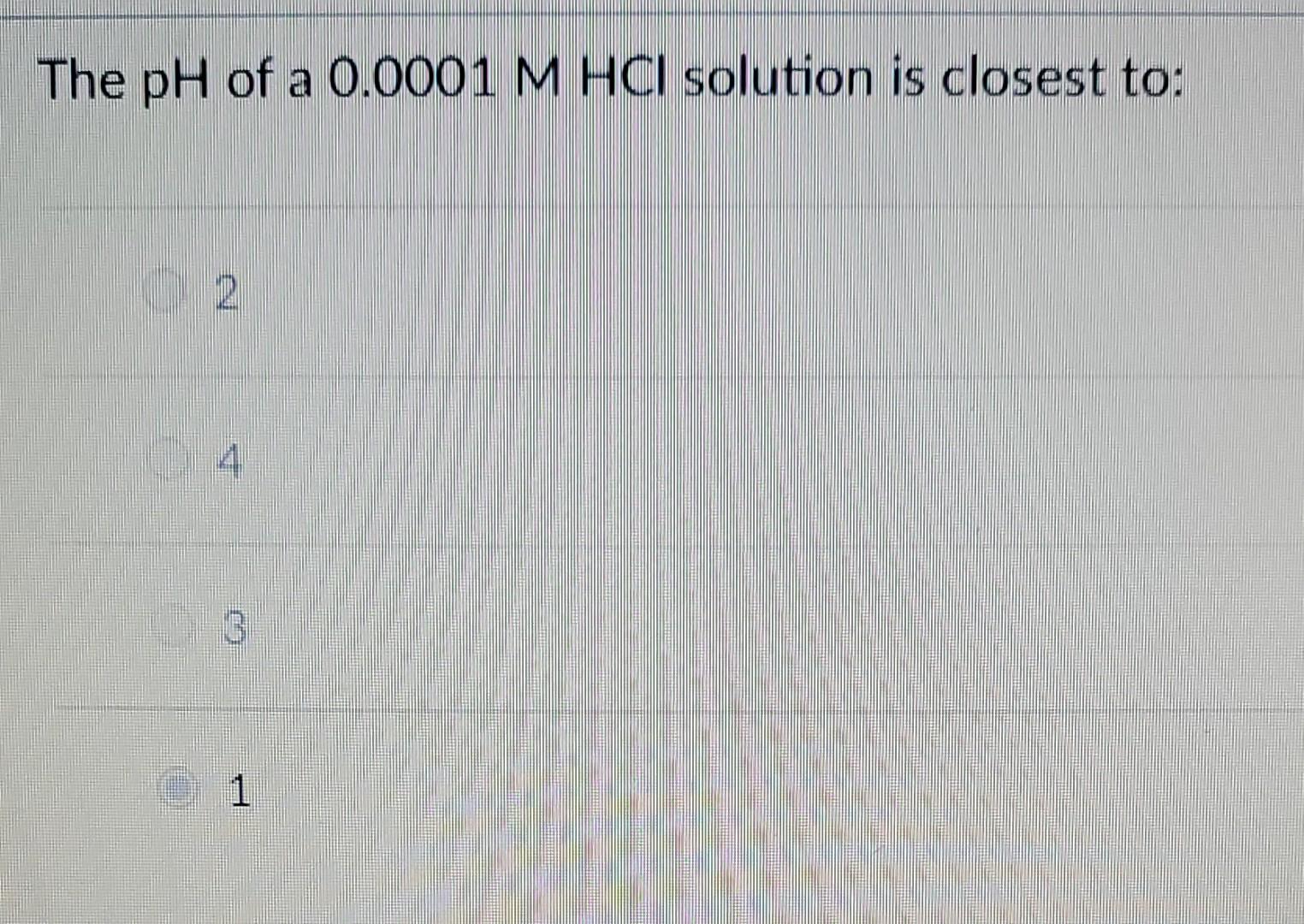
Solved The pH of a 0.0001MHCl solution is closest to 2 4 3
Our expert help has broken down your. What is the ph of a soution containing.0001 m hcl? What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? How ph value is increased when hcl acid solution is diluted. The ph of a solution is determined by taking the negative.
pH of 1M HCl i
What is the ph of 0.0001m hcl solution? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! The ph of a solution is determined by taking the negative log of the.
Solved Help Please! 1)Explain The Reasons For The Differe...
Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. The ph of 0.0001 n solution of koh will be: What is the ph of a soution containing.0001 m hcl? Our.
what is the pH of 0.0001 M HCl solution
Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. A lab assistant prepared a solution by adding a. What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? What is the ph of a soution containing.0001 m.
SOLVED Calculate the pOH of a 0.0001 M HCl solution (Express your
What is the ph of a 0.0001m hcl solution? Our expert help has broken down your. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of the resulting solution when.
The Ph Of 0.0001 N Solution Of Koh Will Be:
Our expert help has broken down your. The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of 0.0001m hcl solution? What is the ph of a soution containing.0001 m hcl?
What Is The Ph Of The Resulting Solution When Equal Volumes Of 0.1 M Naoh And 0.01 M Hcl Are Mixed?
Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. How ph value is increased when hcl acid solution is diluted. A lab assistant prepared a solution by adding a. Aqueous solution of hcl has the ph = 4.
What Is The Ph Of A 0.0001M Hcl Solution?
(remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go!