What Is The Electron Pair Geometry For Xe In Xeo3
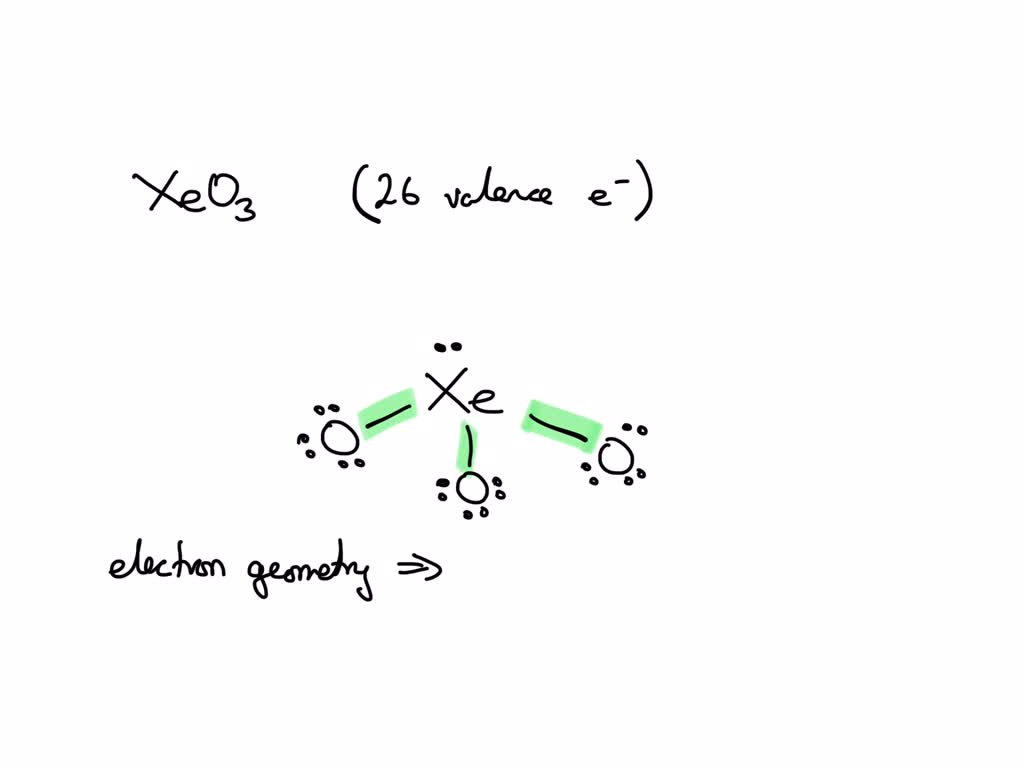
What Is The Electron Pair Geometry For Xe In Xeo3 - The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
Why the bond angle of XeO3 is 103 Chemistry Chemical Bonding and
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
Number of lone pairs of electrons on Xe atoms in XeF2, XeF4 and XeO3
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
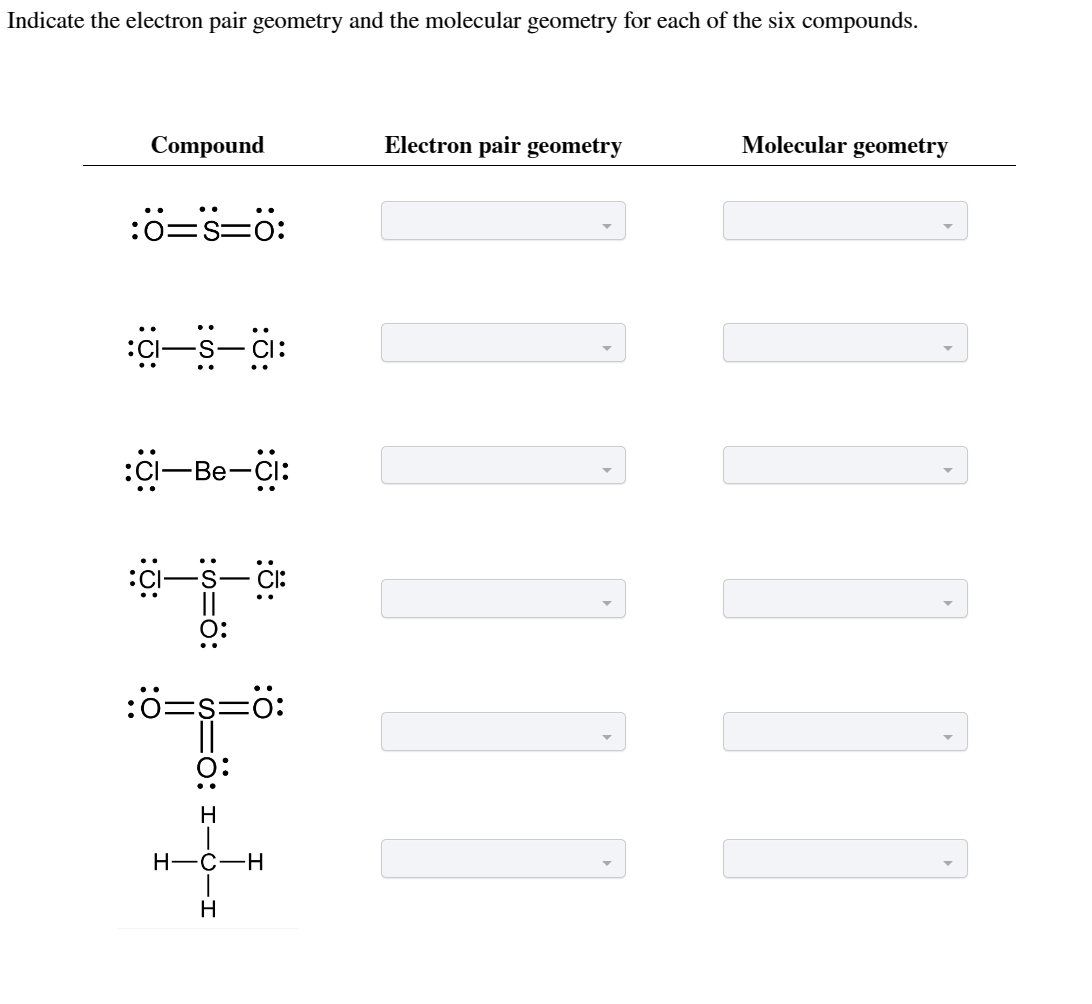
Solved Indicate the electron pair geometry and the molecular
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
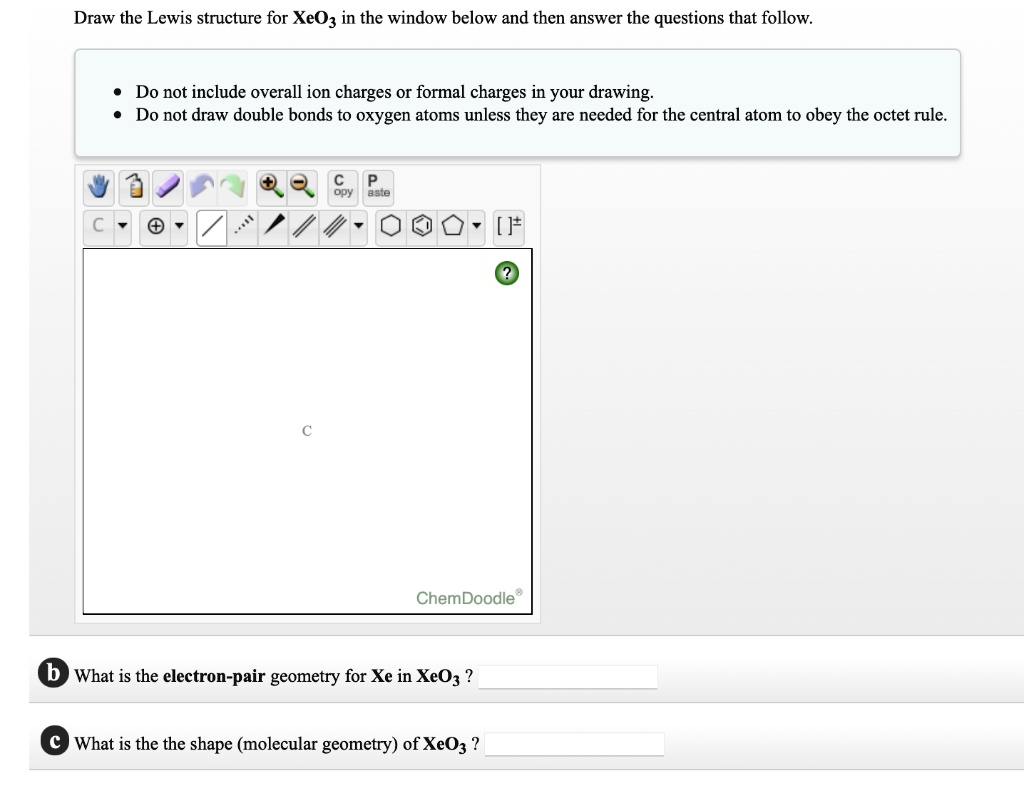
SOLVED Draw the Lewis structure for XeOz in the window below and then
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
[Solved] Predict the electronpair geometry and the molecular structure
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
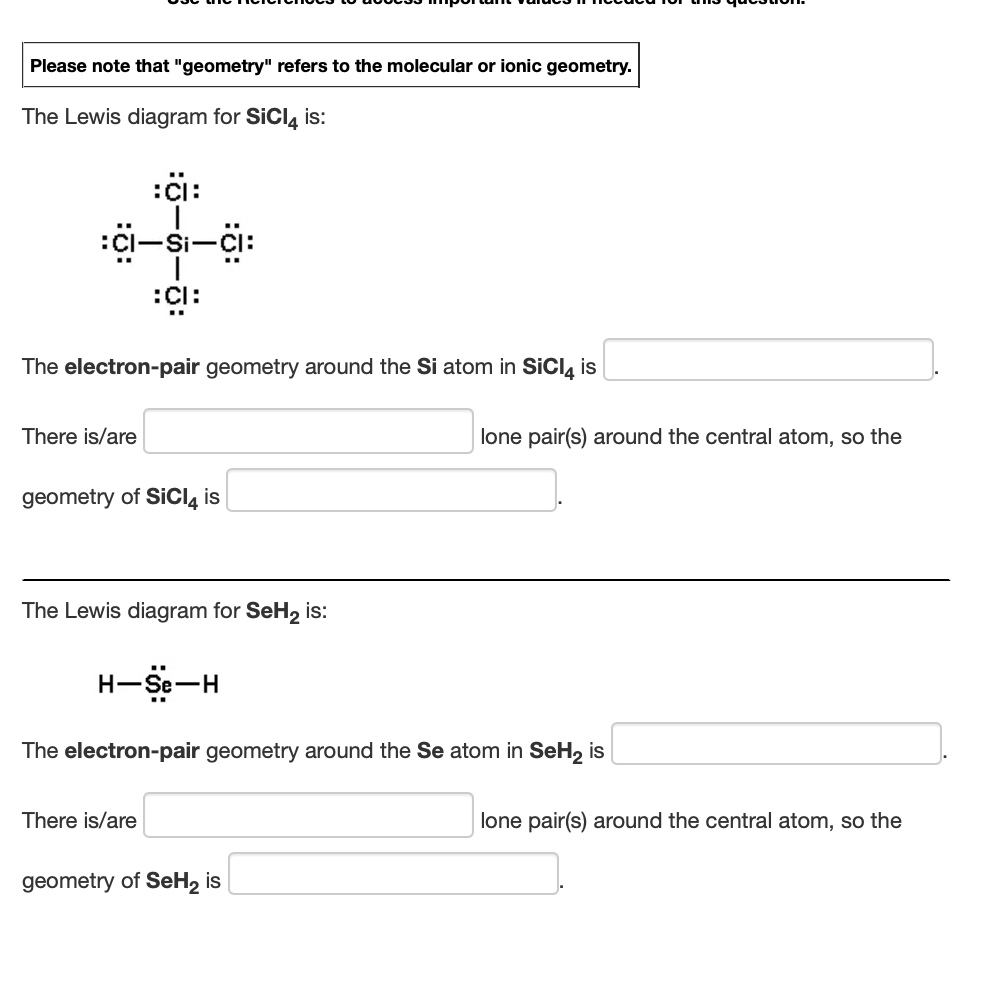
Solved The Lewis diagram for SiCl4 is The electronpair
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
Spatial and electron pair geometry Molecular geometry, Teaching
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
SOLVED Determine the electron geometry (eg), molecular geometry (mg
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
Shape of Molecules VSEPR Theory Affect Shape Of The Molecule
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
There Are Lone Pair(S) Around The Central Atom, So The Molecular Geometry (Shape) Of Po(Oh) 3 Is.
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.