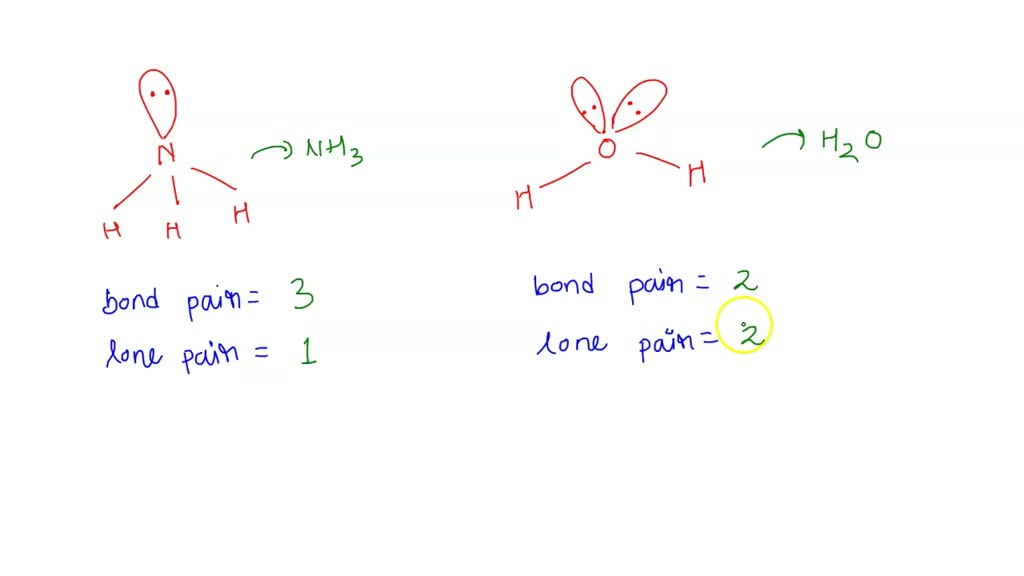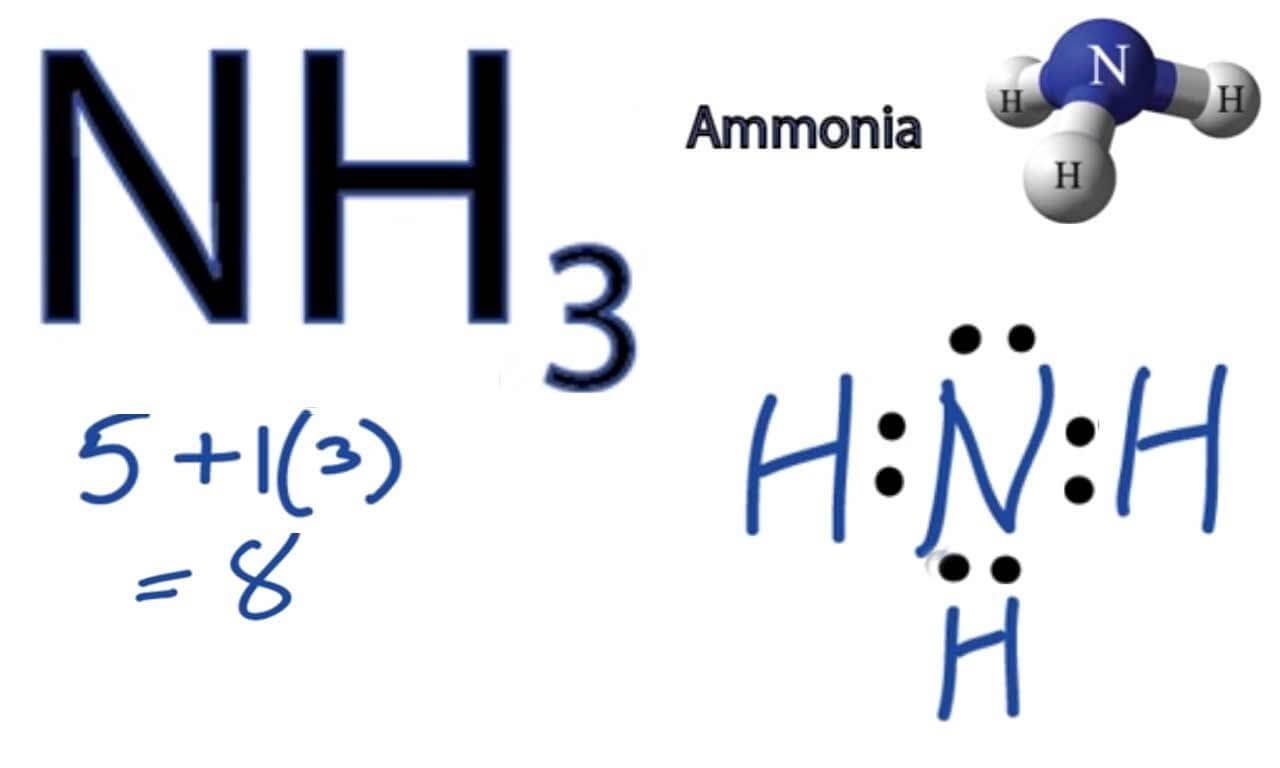What Is The Bond Angle Of Nh3
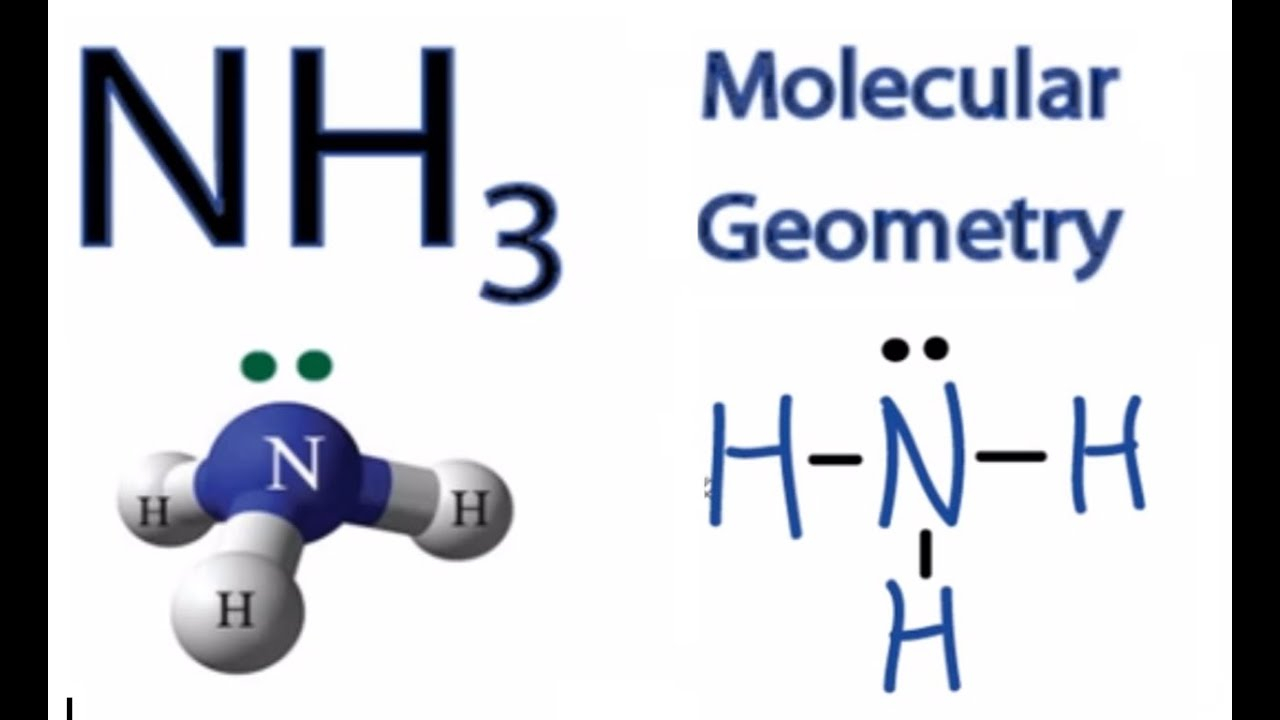
What Is The Bond Angle Of Nh3 - In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,.
Lewis Diagram Of Nh3
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Q1. arrange the following in the increasing order of bond angle nh3 nf3
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
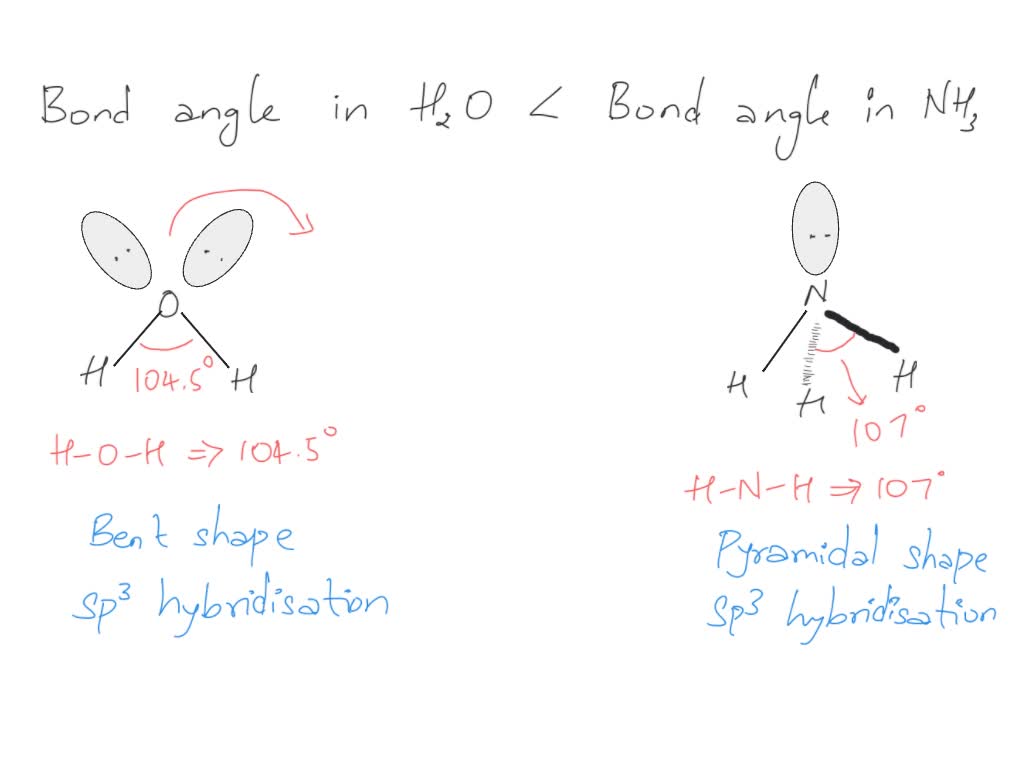
ii) Bond angle of NH3 is than H2O. Justify
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
SOLVED Bond angle in nh3 is greater than bond angle in a s h 3
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
which is the incerrect about bond angles ? (1) NH3 > NF3 (2) NF3 > PF3
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Nh3 é Polar Ou Apolar
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
Why does NH3 have a larger bond angle than PH3?
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
SOLVED Bond angle in Nh3 is more then in H2O . justify
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Nh3 Molecular Geometry Hybridization Bond Angle And Molecular Shape
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
Solved Examine The Bond Lengths And Bond Angles For Three, 42 OFF
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
Nh_3 Has A Bond Angle Of About 106.67^@, While Ph_3 Has A Bond Angle Of About 93.3^@, According To Cccbdb.
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.