What Is Always True Of A Weak Acid
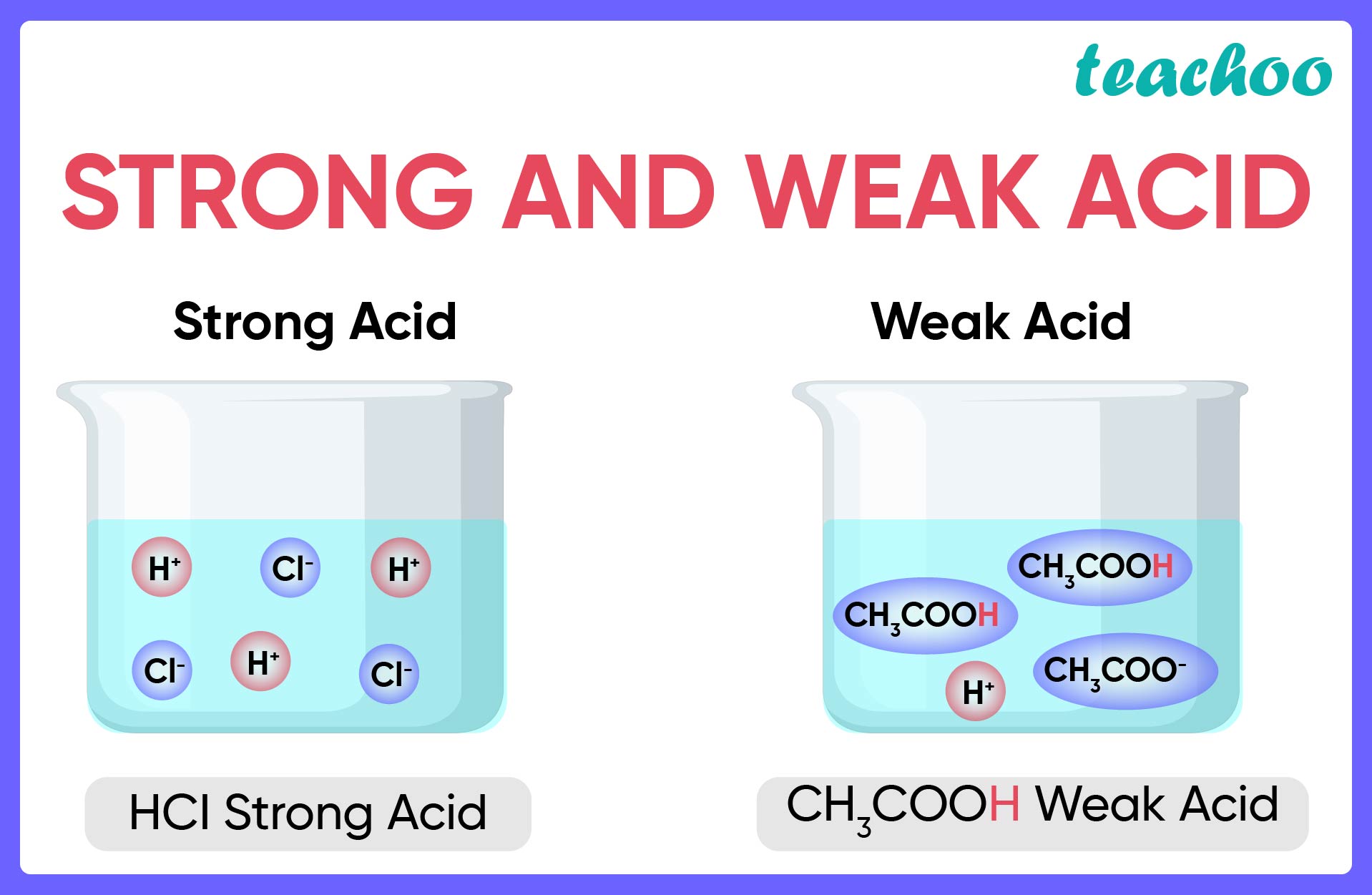
What Is Always True Of A Weak Acid - • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions.
• you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions.
A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come.
Weak Acids Introduction to Chemistry
• so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion.
List of Common Strong and Weak Acids
• so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion.
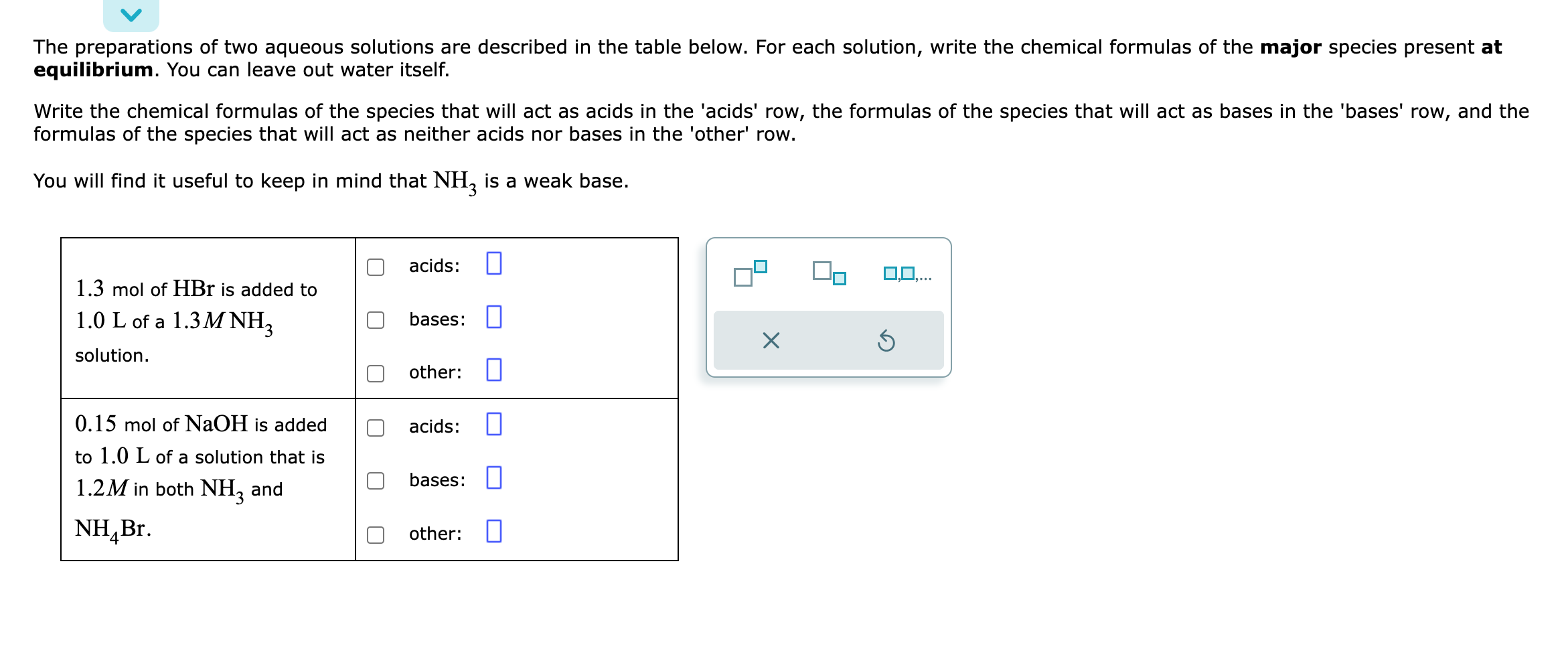
Solved Identifying the major species in weak acid or weak
• you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions.
اسید قوی چیست و چه تفاوتی با اسید ضعیف دارد؟ شیمی یار
A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come.
Strong Acid calculations Weak Acid calculations ppt download
• you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • so where does this ion come.
Weak acids
A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come.
Strong Acid and Weak Acid
• you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • so where does this ion come.
AcidBase Equilibrium How to determine pH and Degree of Dissociation
• you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions.
Solved You will observe a weak acidstrong base titration in
A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come.
What Doesn’t Kill You Makes You Stronger Not Always True The Issue
• you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion. • so where does this ion come. A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions.
• So Where Does This Ion Come.
A weak acid is an acid that partially dissociates into its ions in an aqueous solution, resulting in a relatively low concentration of hydrogen ions. • you may have noticed that the conjugate base of a weak acid, or the conjugate acid of a weak base is always an ion.

/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)