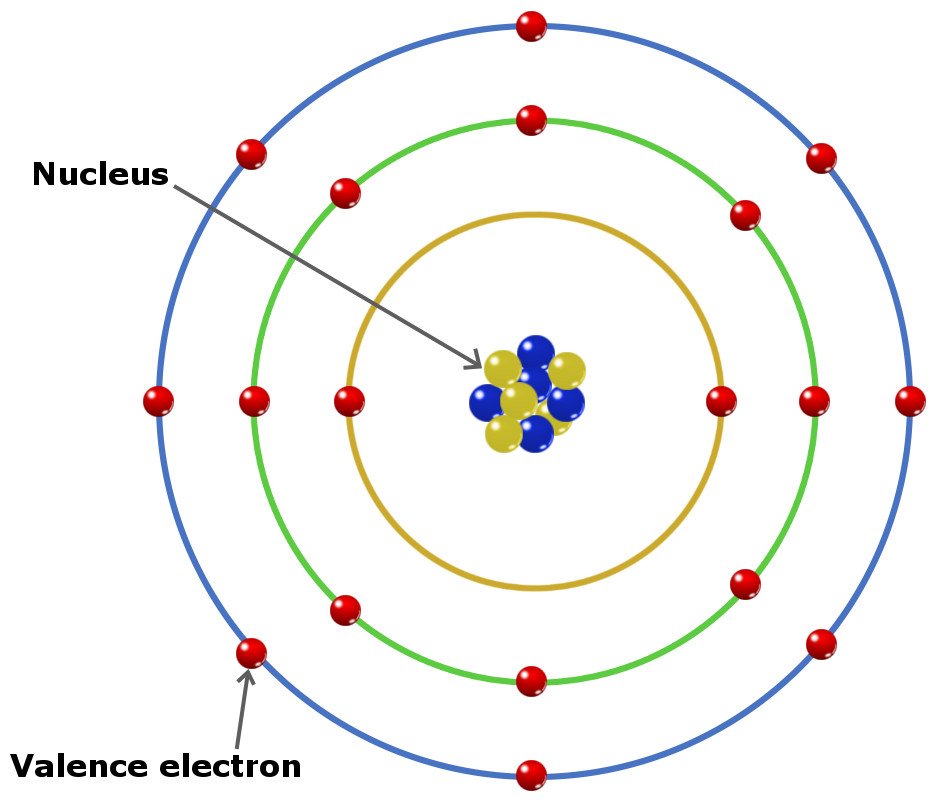
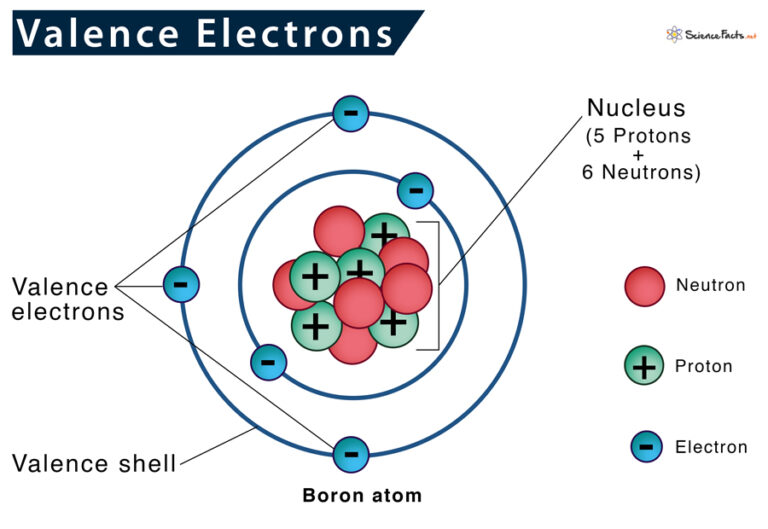
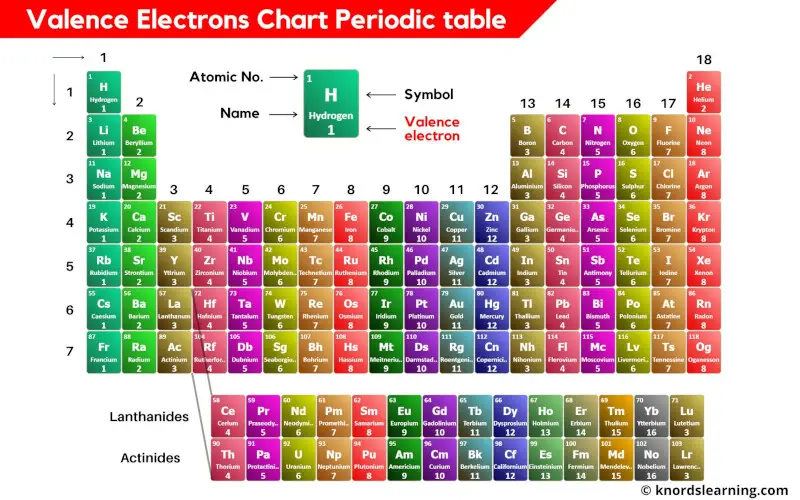
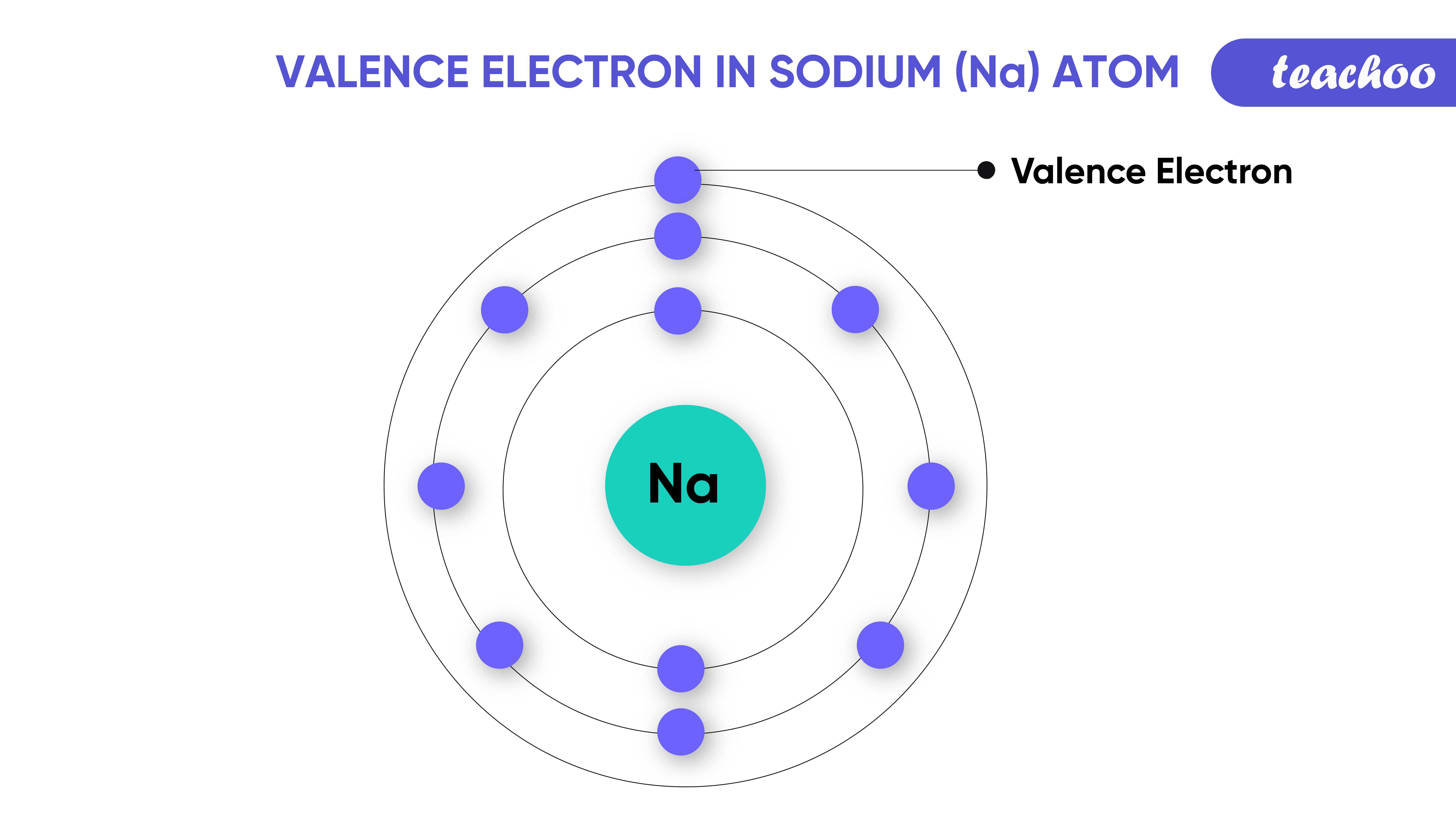
What Elements Have The Same Number Of Valence Electrons
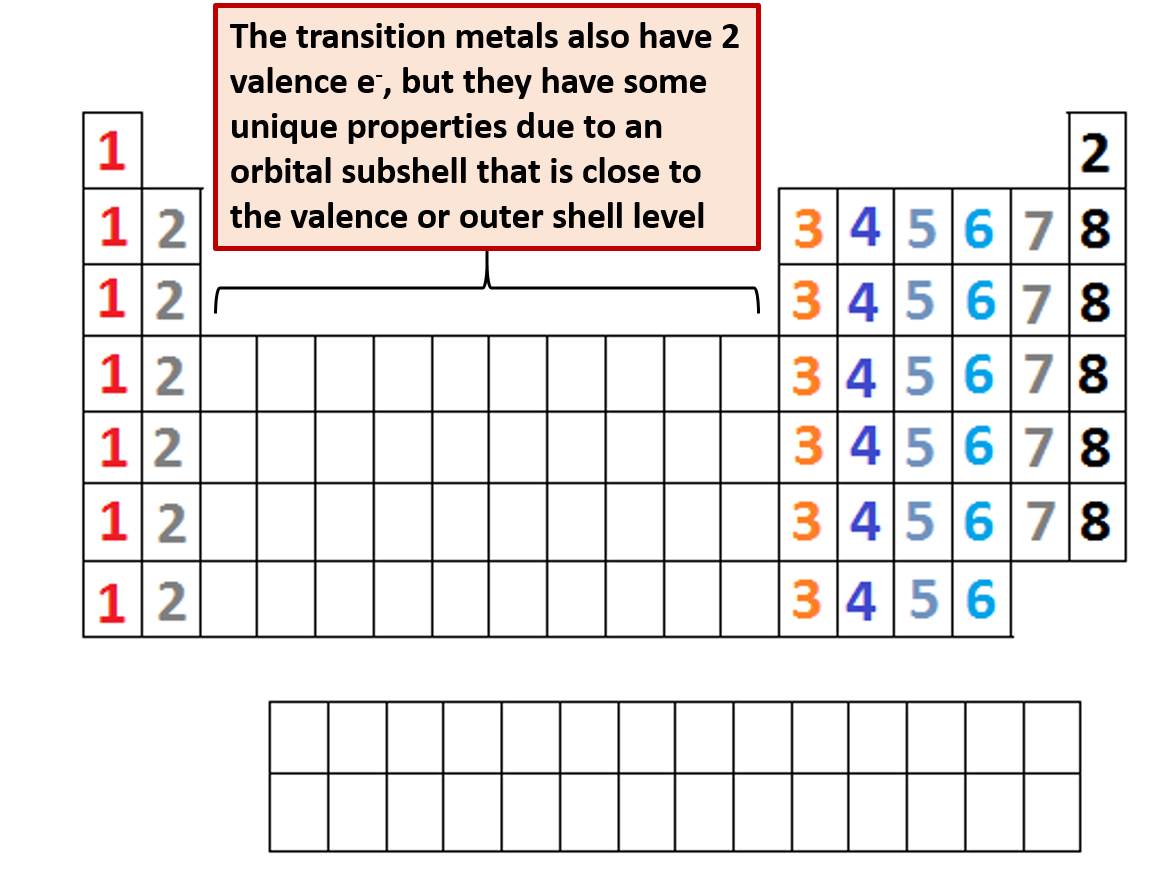
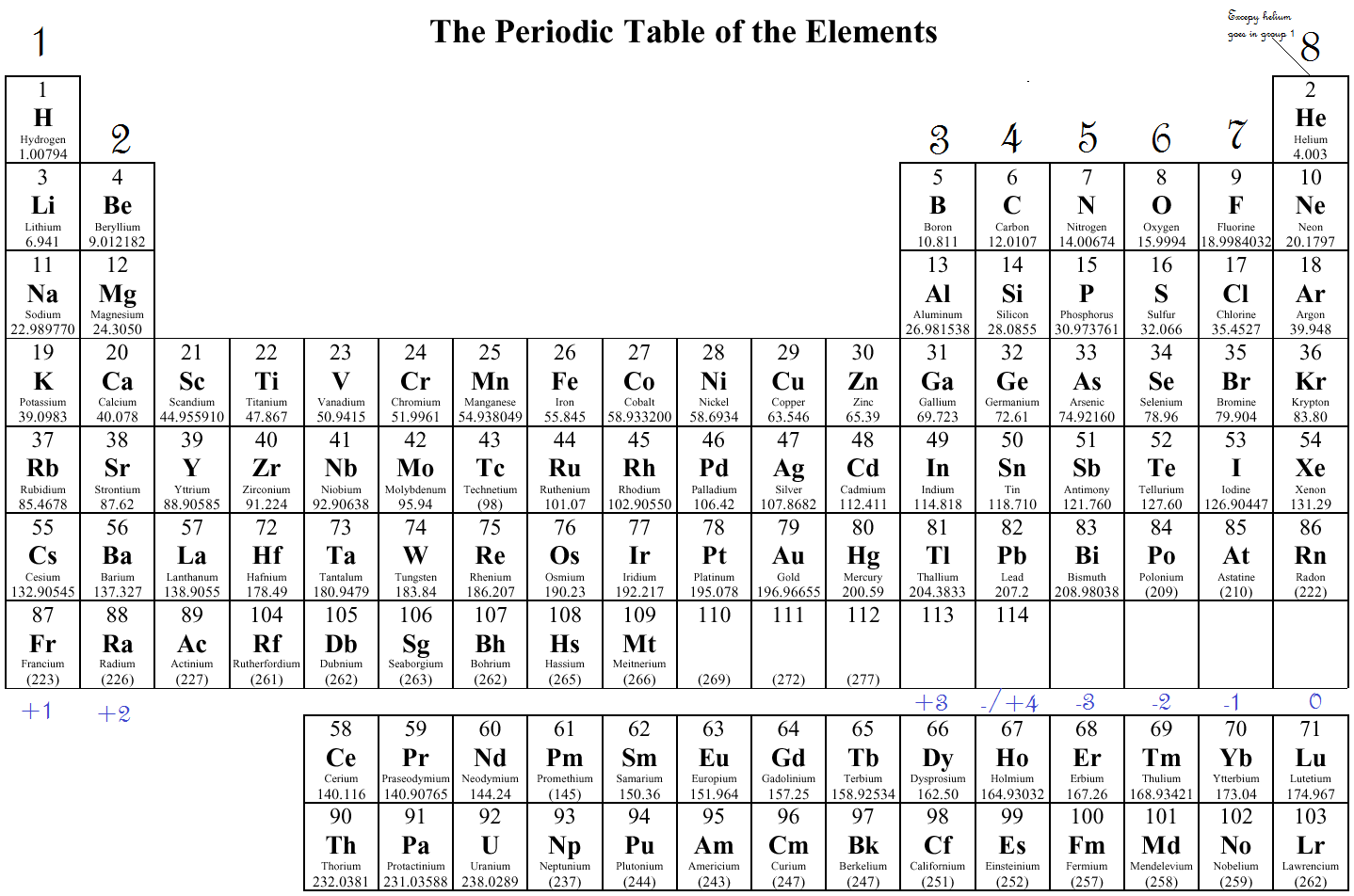
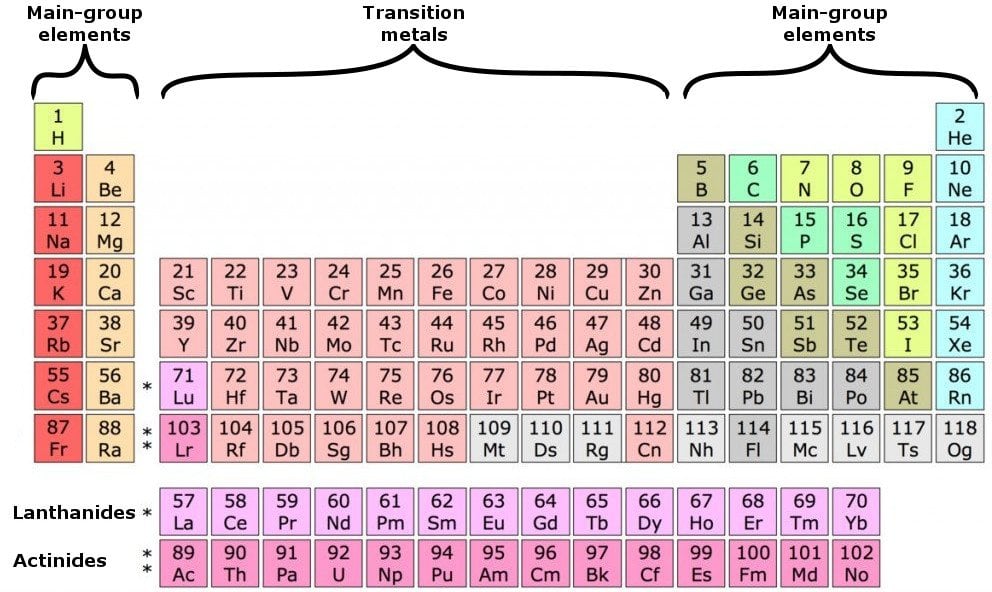
What Elements Have The Same Number Of Valence Electrons - All elements in a group have the same number of valence electrons equal to the first digit of their group number. For example, 1st group of. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. Generally, elements in groups 1,.
Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. For example, 1st group of. All elements in a group have the same number of valence electrons equal to the first digit of their group number. Generally, elements in groups 1,.
For example, 1st group of. All elements in a group have the same number of valence electrons equal to the first digit of their group number. Generally, elements in groups 1,. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table.
How To Find Valence Number
For example, 1st group of. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. All elements in a group have the same number of valence electrons equal to the first digit of their group number. Generally, elements in groups 1,.
Valence Electrons Definition, Location, Importance, and Diagram
All elements in a group have the same number of valence electrons equal to the first digit of their group number. Generally, elements in groups 1,. For example, 1st group of. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table.
What Are Valence Electrons? Definition and Periodic Table Electrons
Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. All elements in a group have the same number of valence electrons equal to the first digit of their group number. For example, 1st group of. Generally, elements in groups 1,.
Valence Electrons Chart of Elements (With Periodic table)
Generally, elements in groups 1,. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. All elements in a group have the same number of valence electrons equal to the first digit of their group number. For example, 1st group of.
How To Identify The Valence Electron
Generally, elements in groups 1,. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. For example, 1st group of. All elements in a group have the same number of valence electrons equal to the first digit of their group number.
How Many Valence Electrons Do Rare Earth Elements Have The Earth
For example, 1st group of. Generally, elements in groups 1,. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. All elements in a group have the same number of valence electrons equal to the first digit of their group number.
Enriched Chemistry Compound Project Valence Electrons And How They Are
All elements in a group have the same number of valence electrons equal to the first digit of their group number. Generally, elements in groups 1,. For example, 1st group of. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table.
What Are Valence Electrons And How To Find Them? Where Are They Located?
Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. Generally, elements in groups 1,. For example, 1st group of. All elements in a group have the same number of valence electrons equal to the first digit of their group number.
What Are Valence Electrons? Definition and Periodic Table
Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. All elements in a group have the same number of valence electrons equal to the first digit of their group number. For example, 1st group of. Generally, elements in groups 1,.
A Semiconductor Has How Many Valence Electrons? New
For example, 1st group of. Generally, elements in groups 1,. Elements whose atoms have the same number of valence electrons are grouped together in the periodic table. All elements in a group have the same number of valence electrons equal to the first digit of their group number.
Elements Whose Atoms Have The Same Number Of Valence Electrons Are Grouped Together In The Periodic Table.
For example, 1st group of. All elements in a group have the same number of valence electrons equal to the first digit of their group number. Generally, elements in groups 1,.