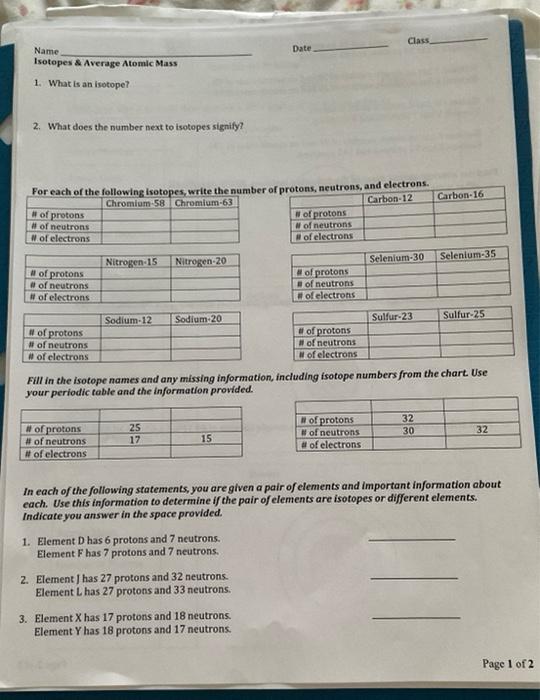
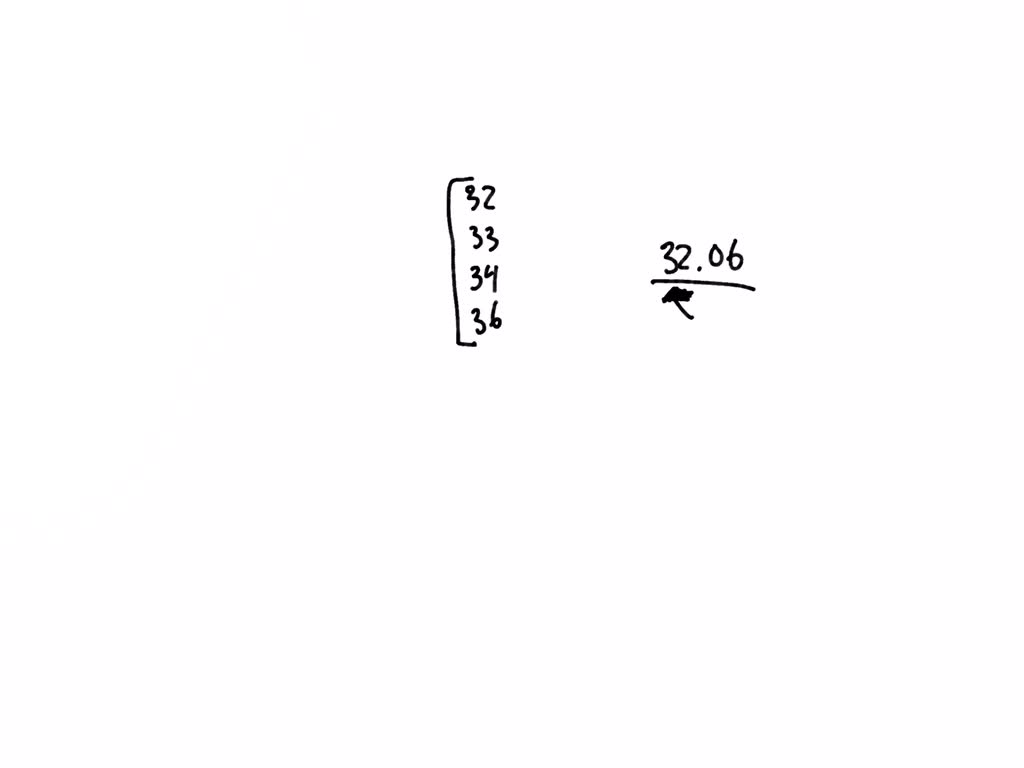
What Does The Number Next To Isotopes Signify
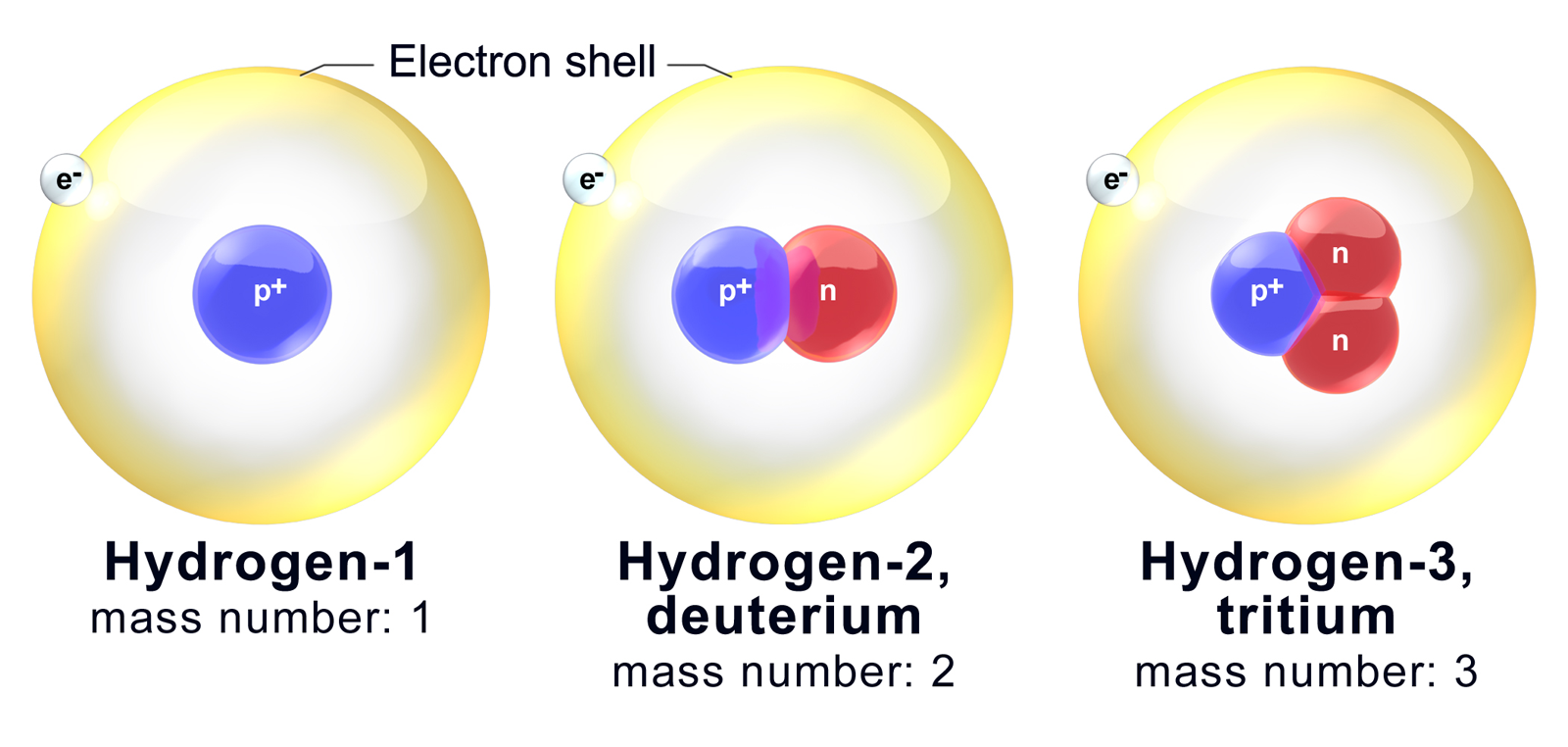
What Does The Number Next To Isotopes Signify - Isotopes are versions of the same element. What does the number next to isotopes signify?(eg. What does the number next to isotopes signify? The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. Isotopes of an element have the same. The number indicates the isotope's mass number. The number next to isotopes signifies the mass number of the isotope. Isotopes are versions of the same element. Become a member and unlock all study.
Become a member and unlock all study. Isotopes are versions of the same element. The number next to isotopes signifies the mass number of the isotope. Isotopes are versions of the same element. The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. What does the number next to isotopes signify?(eg. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. The number indicates the isotope's mass number. Isotopes of an element have the same. It is called the mass number.
The number next to isotopes signifies the mass number of the isotope. Isotopes of an element have the same. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. It is called the mass number. Become a member and unlock all study. What does the number next to isotopes signify?(eg. Isotopes are versions of the same element. The number indicates the isotope's mass number. What does the number next to isotopes signify?
PPT Isotopes PowerPoint Presentation, free download ID2481748
The number indicates the isotope's mass number. It is called the mass number. Become a member and unlock all study. Isotopes are versions of the same element. What does the number next to isotopes signify?
Isotopes Practice Worksheet Ions And Isotopes Practice Two Versions
The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. Isotopes of an element have the same. What does the number next to isotopes signify? The number next to isotopes signifies the mass number of the isotope. The number indicates the isotope's mass number.
Answered 1. What is an isotope? 2. What does the… bartleby
They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. What does the number next to isotopes signify?(eg. The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. Become a member and unlock all study. What does the number.
Practice Isotope Calculations 1 Worksheet Answers / Isotopes And Ions
Isotopes are versions of the same element. Isotopes of an element have the same. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. The number next to isotopes signifies the mass number of the isotope. Isotopes are versions of the same element.
DOE Explains...Isotopes Department of Energy
What does the number next to isotopes signify?(eg. What does the number next to isotopes signify? The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. It is called the mass number. The number indicates the isotope's mass number.
Solved 2. What does the number next to isotopes signify? For
What does the number next to isotopes signify? The number next to isotopes signifies the mass number of the isotope. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. The number indicates the isotope's mass number. Isotopes are versions of the same element.
Element Notation Examples
The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. It is called the mass number. Isotopes are versions of the same element. The number next to isotopes signifies the mass number of the isotope. They have the same number of protons and electrons as the element but different mass numbers.
SOLVED one of the isotopes of sulfur is represented as sulfur 35 or S
The number indicates the isotope's mass number. What does the number next to isotopes signify?(eg. Isotopes are versions of the same element. Isotopes of an element have the same. The number next to isotopes signifies the mass number of the isotope.
What is the isotope? Yoors
Become a member and unlock all study. The number indicates the isotope's mass number. The number next to isotopes signifies the mass number of the isotope. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. It is called the mass number.
Solved 2. What does the number next to isotopes signify? For
They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.what does the. The number indicates the isotope's mass number. The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. What does the number next to isotopes signify? Become a member and.
They Have The Same Number Of Protons And Electrons As The Element But Different Mass Numbers And Number Of Neutrons.what Does The.
Become a member and unlock all study. Isotopes are versions of the same element. What does the number next to isotopes signify?(eg. Isotopes of an element have the same.
Isotopes Are Versions Of The Same Element.
The number next to isotopes signifies the mass number of the isotope. What does the number next to isotopes signify? The number next to isotopes represents the sum of the protons and neutrons in the nucleus of the atom. It is called the mass number.