What Does The Dotted Line Between The Water Molecules Represent
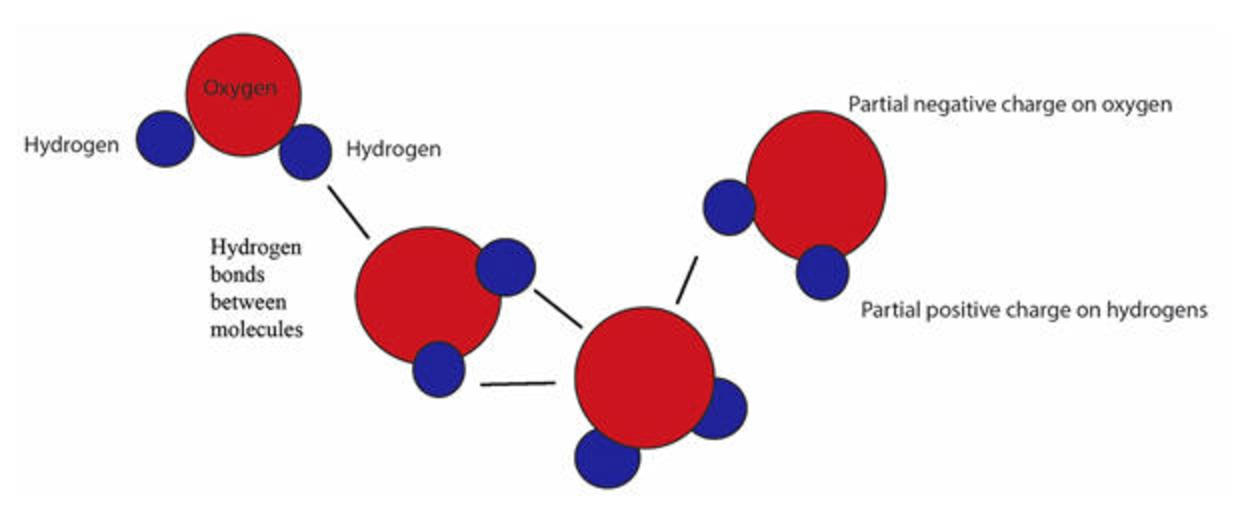
What Does The Dotted Line Between The Water Molecules Represent - A polar bond a covalent bond a. The diagram shows two water molecules. Each water molecule is hydrogen bonded to four. In the case of water, hydrogen bonds form between. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. What does the dotted line between the water molecules represent? The force of attraction, shown here as a dotted line, is called a hydrogen bond. Hydrogen bonds shown as the dotted lines between water molecules. This type of bond is a special. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen.
The force of attraction, shown here as a dotted line, is called a hydrogen bond. In the case of water, hydrogen bonds form between. Hydrogen bonds shown as the dotted lines between water molecules. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. This type of bond is a special. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. What does the dotted line between the water molecules represent? Each water molecule is hydrogen bonded to four. A polar bond a covalent bond a. The diagram shows two water molecules.
The diagram shows two water molecules. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. Hydrogen bonds shown as the dotted lines between water molecules. Each water molecule is hydrogen bonded to four. In the case of water, hydrogen bonds form between. The force of attraction, shown here as a dotted line, is called a hydrogen bond. This type of bond is a special. A polar bond a covalent bond a. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. What does the dotted line between the water molecules represent?
Nature up close Water molecules CBS News
A polar bond a covalent bond a. This type of bond is a special. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. In the case of water, hydrogen bonds form between.
The strong polar bond between water molecules creates water cohesion.
Hydrogen bonds shown as the dotted lines between water molecules. In the case of water, hydrogen bonds form between. Each water molecule is hydrogen bonded to four. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. What does the dotted line between the water molecules represent?
Solved The diagram shows two water molecules. What does the dotted
The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. A polar bond a covalent bond a. In the case of water, hydrogen bonds form between. Hydrogen bonds shown as the dotted lines between water molecules. The force of attraction, shown here as a dotted line, is called a hydrogen bond.
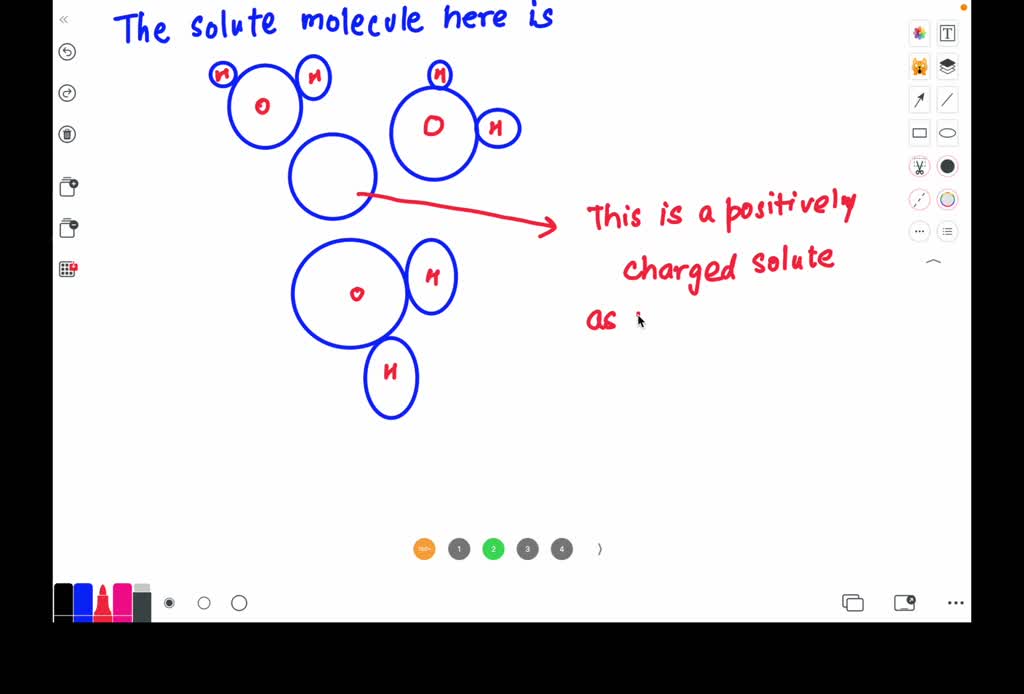
Task Draw water molecules interacting with each possible p.pdf
The force of attraction, shown here as a dotted line, is called a hydrogen bond. In the case of water, hydrogen bonds form between. Each water molecule is hydrogen bonded to four. What does the dotted line between the water molecules represent? Hydrogen bonds shown as the dotted lines between water molecules.
Hydrogen bonding Hopinno
Hydrogen bonds shown as the dotted lines between water molecules. The force of attraction, shown here as a dotted line, is called a hydrogen bond. What does the dotted line between the water molecules represent? In the case of water, hydrogen bonds form between. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond.
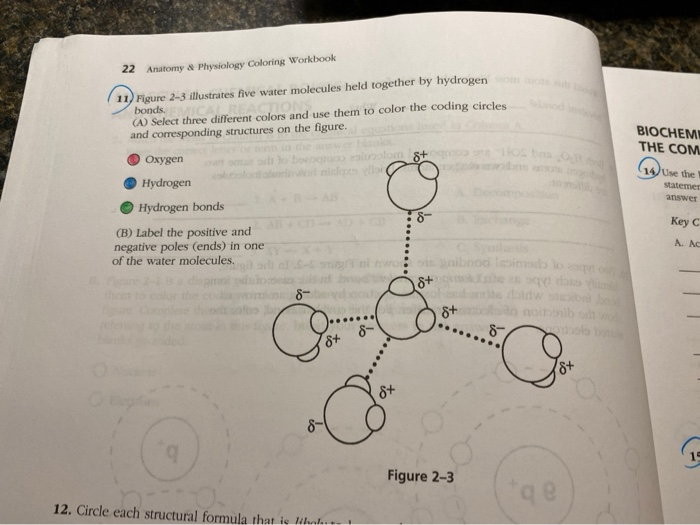
Solved 11 Figure 23 illustrates five water molecules held
The force of attraction, shown here as a dotted line, is called a hydrogen bond. The diagram shows two water molecules. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. In the case of water, hydrogen bonds form between. Each water molecule is hydrogen bonded to four.
SOLVED 17) Based on your knowledge of the polarity of water molecules
The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. Hydrogen bonds shown as the dotted lines between water molecules. This type of bond is a special. A polar bond a covalent bond a. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond.
Best Of How Is Water Molecule Like A
Hydrogen bonds shown as the dotted lines between water molecules. Each water molecule is hydrogen bonded to four. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. The force of attraction, shown here as a dotted line, is called a hydrogen bond. In the case of water, hydrogen bonds form between.
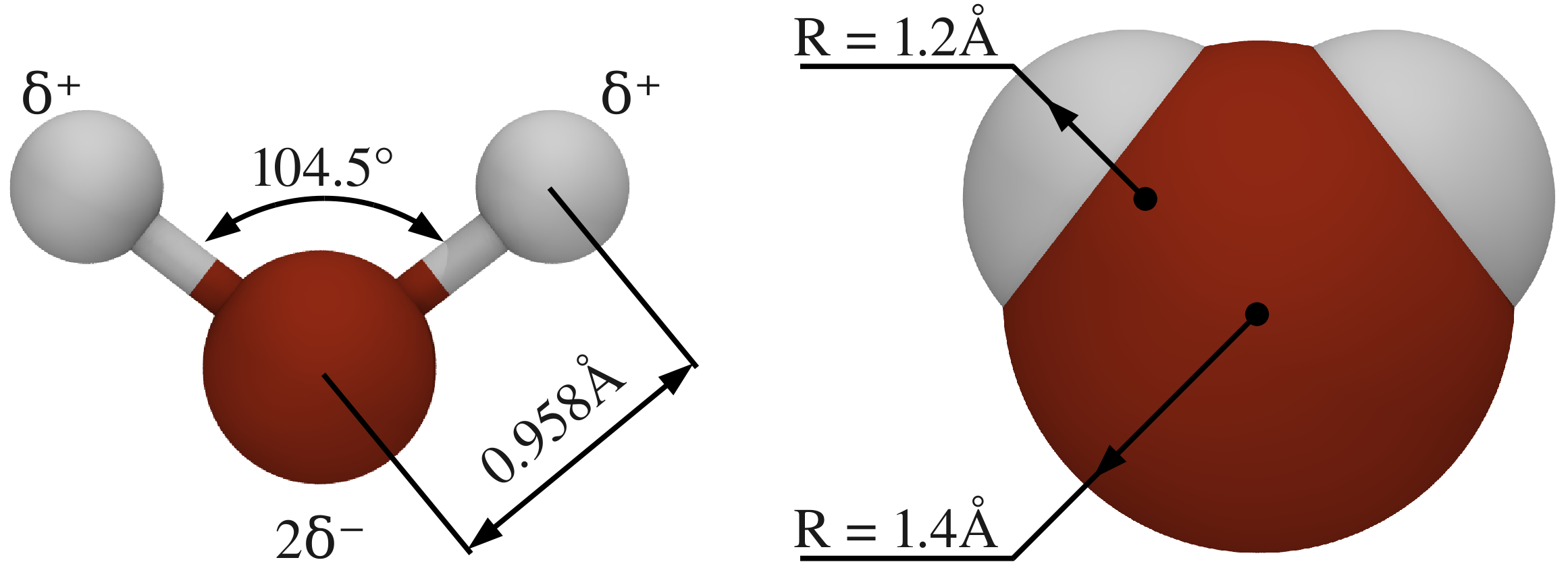
Water molecule — MD simulations documentation
A polar bond a covalent bond a. This type of bond is a special. Hydrogen bonds shown as the dotted lines between water molecules. The force of attraction, shown here as a dotted line, is called a hydrogen bond. The diagram shows two water molecules.
[Solved] Draw five molecules of water (check out figure 2.10 in your
In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. What does the dotted line between the water molecules represent? Hydrogen bonds shown as the dotted lines between water molecules. Each water molecule is hydrogen bonded to four. The force of attraction, shown here as a dotted line, is called a hydrogen bond.
The Dotted Line Between Water Molecules Represents A Hydrogen Bond, Which Forms Between The Partially Positive Hydrogen.
A polar bond a covalent bond a. This type of bond is a special. Each water molecule is hydrogen bonded to four. The force of attraction, shown here as a dotted line, is called a hydrogen bond.
The Diagram Shows Two Water Molecules.
What does the dotted line between the water molecules represent? In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. In the case of water, hydrogen bonds form between. Hydrogen bonds shown as the dotted lines between water molecules.