What Distinguishes Strong Acids From Weak Acids
What Distinguishes Strong Acids From Weak Acids - Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low.
Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low.
Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions.
Difference between Strong and Weak Acids [in Table Form] Teachoo
Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low.
Strong and weak acids collection with educational diagram outline
Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Weak acids have a low.
Learn All About The Strong Acids and Bases PraxiLabs
Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low.
Learning goals Students will be able to ppt download
Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low.
Strong Organic Acids List
Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions.
Acid Strength, Ka, and pKa Chemistry Steps
Weak acids have a low. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration.
SOLVEDa. What distinguishes strong acids from weak acids? b. Give two
Weak acids have a low. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions.
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions
Weak acids have a low. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration.
اسید قوی چیست و چه تفاوتی با اسید ضعیف دارد؟ شیمی یار
Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Strong acids are highly ionizable and completely dissociate in water, resulting in a high concentration of (h+) (h +) ions. Weak acids have a low.
Strong Acids Are Highly Ionizable And Completely Dissociate In Water, Resulting In A High Concentration Of (H+) (H +) Ions.
Strong acids usually pass more current as compared to the weak acids for the same voltage and concentration. Weak acids have a low.
![Difference between Strong and Weak Acids [in Table Form] Teachoo](https://d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/eb34c57a-aeb2-4e7c-aa0b-613a11a6595e/differentiate-between-strong-and-weak-acids-01.jpg)
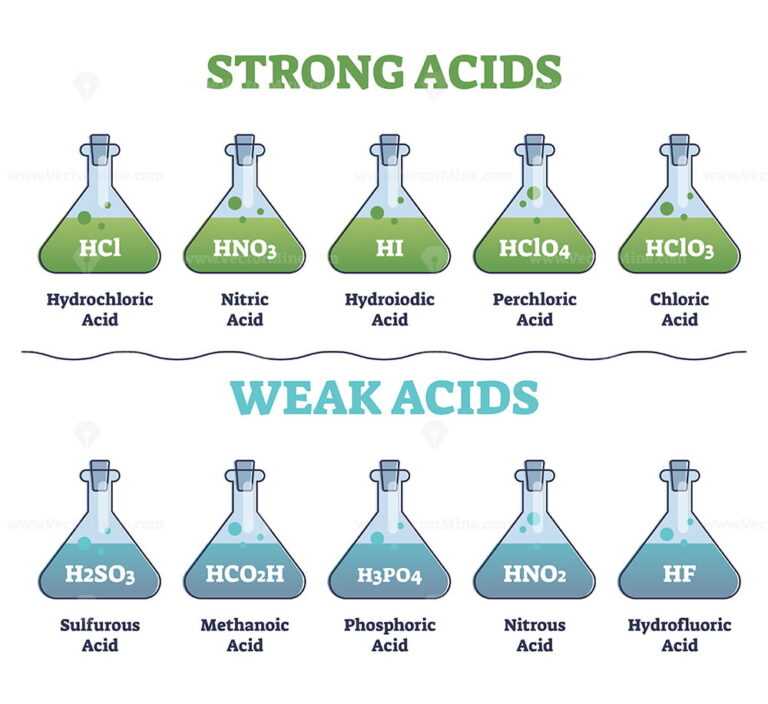
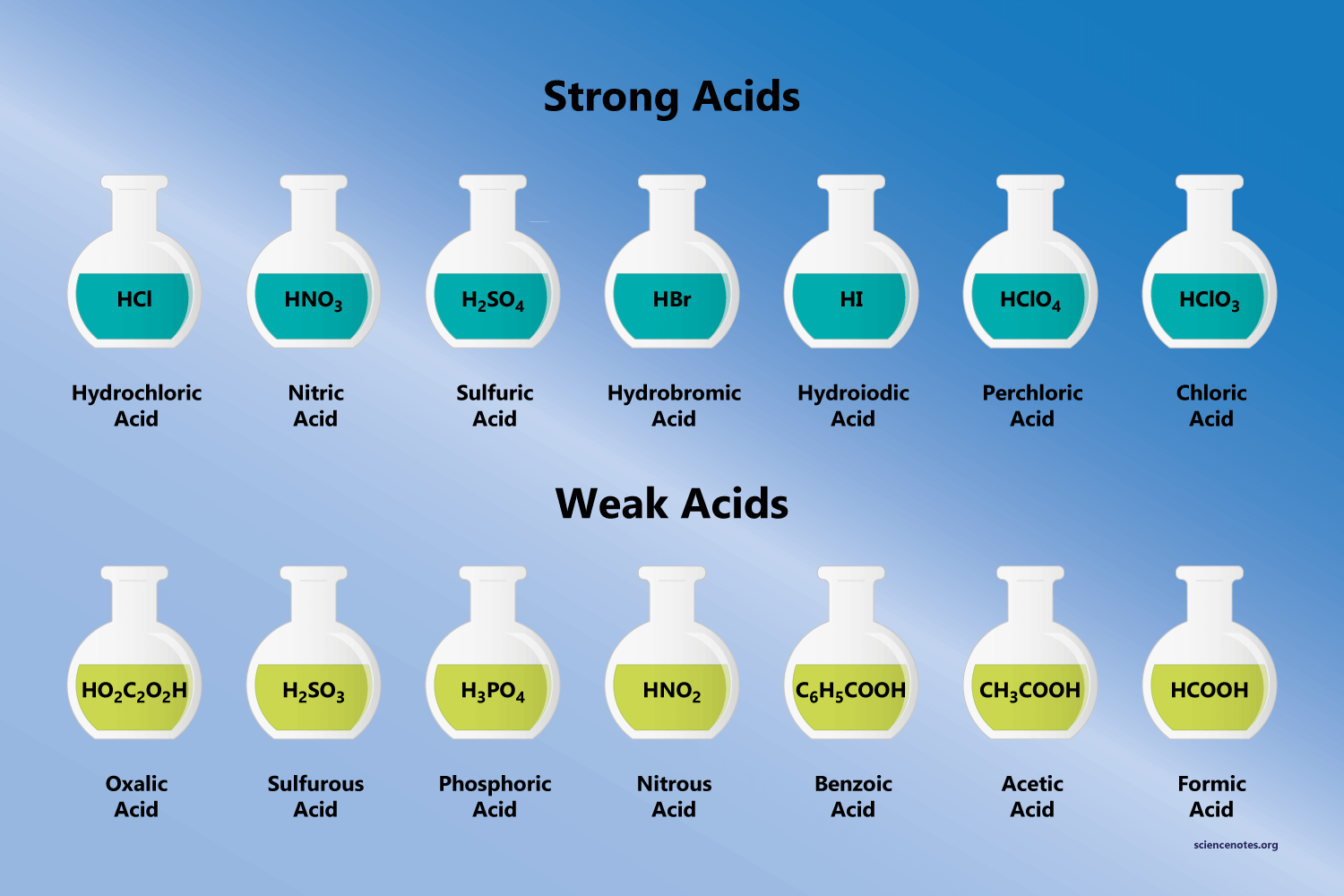


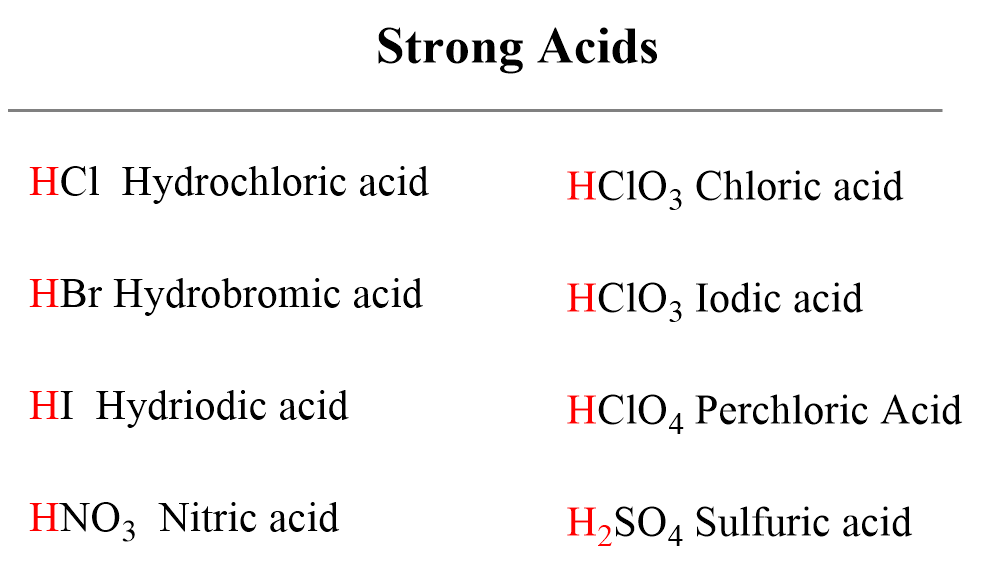


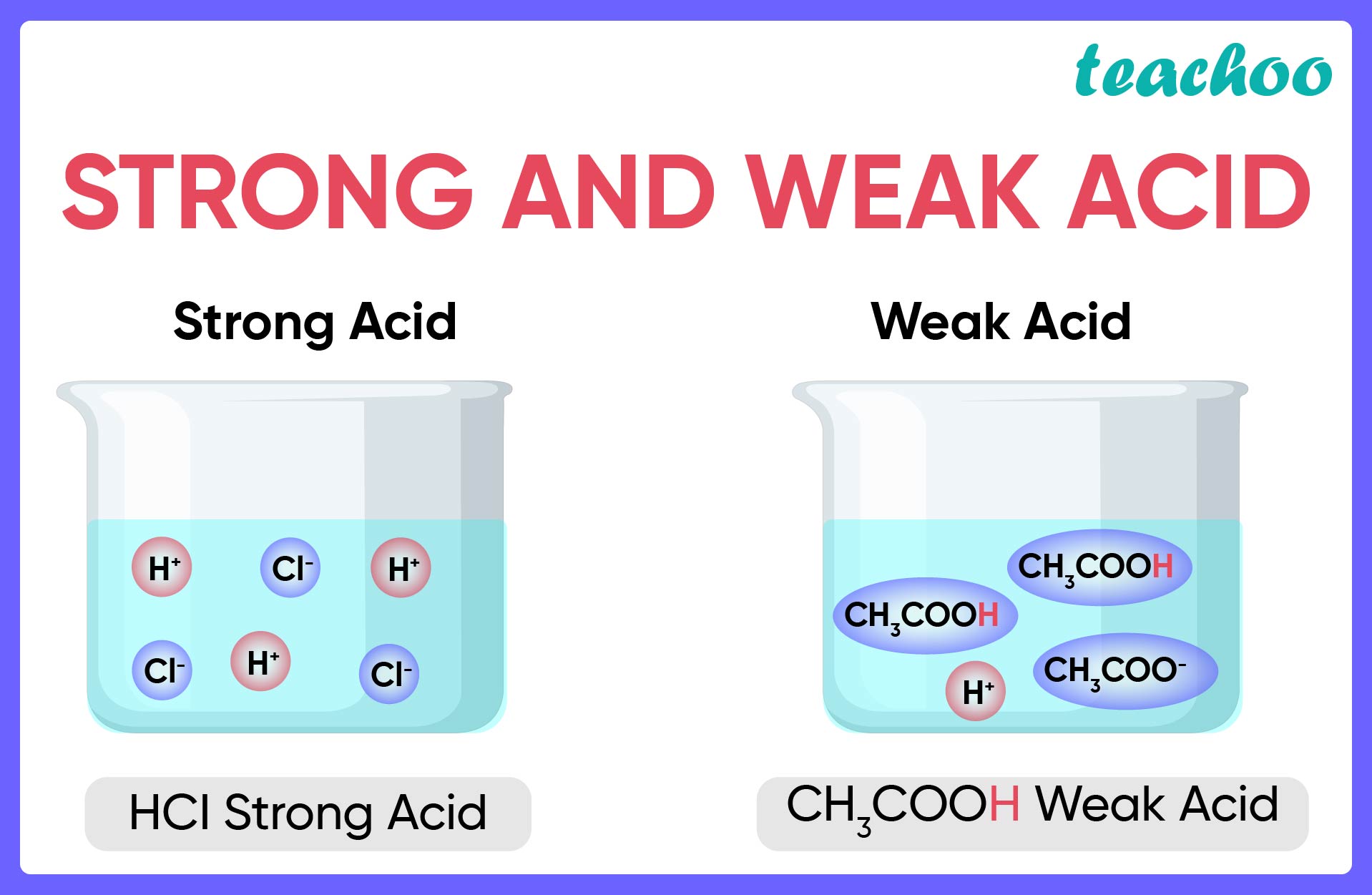
:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)