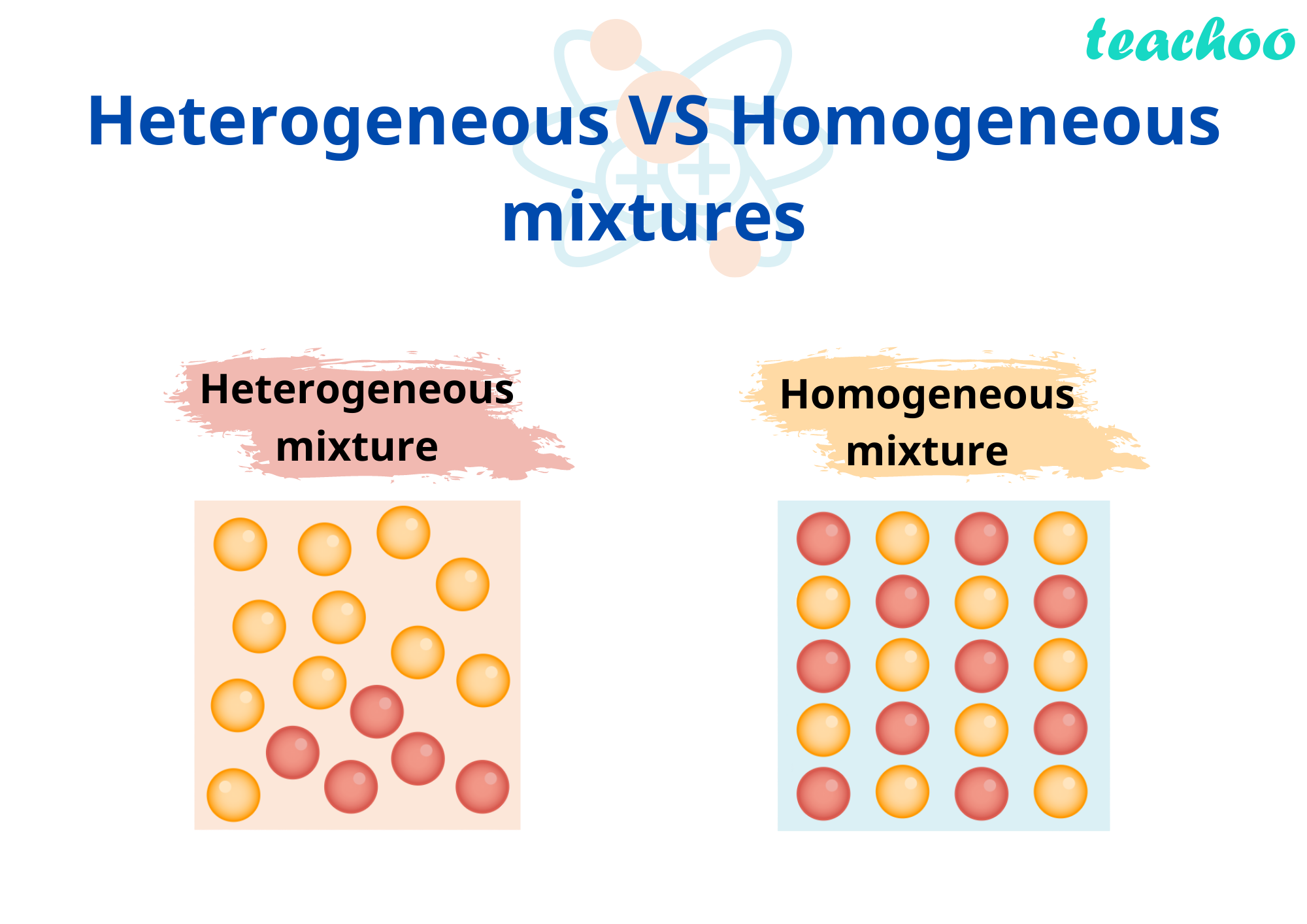
Homogeneous Vs Heterogeneous
Homogeneous Vs Heterogeneous - In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. Heterogeneous systems are characterized by their diversity, lack of uniformity, potential for. Reactions that take place on the surface of a catalyst of a. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. In imaging, a homogeneous appearance indicates uniformity. Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. Heterogeneous and homogeneous systems possess distinct attributes that shape their behavior, interactions, and analysis. A homogeneous mixture is a mixture in which the components. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with its counterpart, homogeneous.
Heterogeneous systems are characterized by their diversity, lack of uniformity, potential for. In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with its counterpart, homogeneous. Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. A homogeneous mixture is a mixture in which the components. Reactions that take place on the surface of a catalyst of a. Heterogeneous and homogeneous systems possess distinct attributes that shape their behavior, interactions, and analysis. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. In imaging, a homogeneous appearance indicates uniformity.
Reactions that take place on the surface of a catalyst of a. In imaging, a homogeneous appearance indicates uniformity. A homogeneous mixture is a mixture in which the components. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. Heterogeneous and homogeneous systems possess distinct attributes that shape their behavior, interactions, and analysis. Heterogeneous systems are characterized by their diversity, lack of uniformity, potential for. In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with its counterpart, homogeneous.
What is a Mixture in Chemistry? The Chemistry Blog
A homogeneous mixture is a mixture in which the components. In imaging, a homogeneous appearance indicates uniformity. In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. Heterogeneous and homogeneous systems possess distinct attributes that shape their behavior, interactions, and.
Difference Between Homogeneous and Heterogeneous Mixtures
In imaging, a homogeneous appearance indicates uniformity. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with its counterpart, homogeneous. Reactions that take place on the surface of a catalyst of a. Heterogeneous systems.
Homogeneous vs heterogeneous mixture physical properties outline
Reactions that take place on the surface of a catalyst of a. Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things.
Difference between Homogeneous and Heterogeneous Mixtures (Homogeneous
Reactions that take place on the surface of a catalyst of a. Heterogeneous systems are characterized by their diversity, lack of uniformity, potential for. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with.
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo
Reactions that take place on the surface of a catalyst of a. In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the.
Differentiate b/w Homogeneous and Heterogeneous mixtures Teachoo
In imaging, a homogeneous appearance indicates uniformity. Reactions that take place on the surface of a catalyst of a. Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. A homogeneous mixture is a mixture in which the components. To grasp the concept of.
Difference Between Homogeneous and Heterogeneous Compare the
Reactions that take place on the surface of a catalyst of a. In imaging, a homogeneous appearance indicates uniformity. Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. A homogeneous mixture is a mixture in which the components. Heterogeneous systems are characterized by.
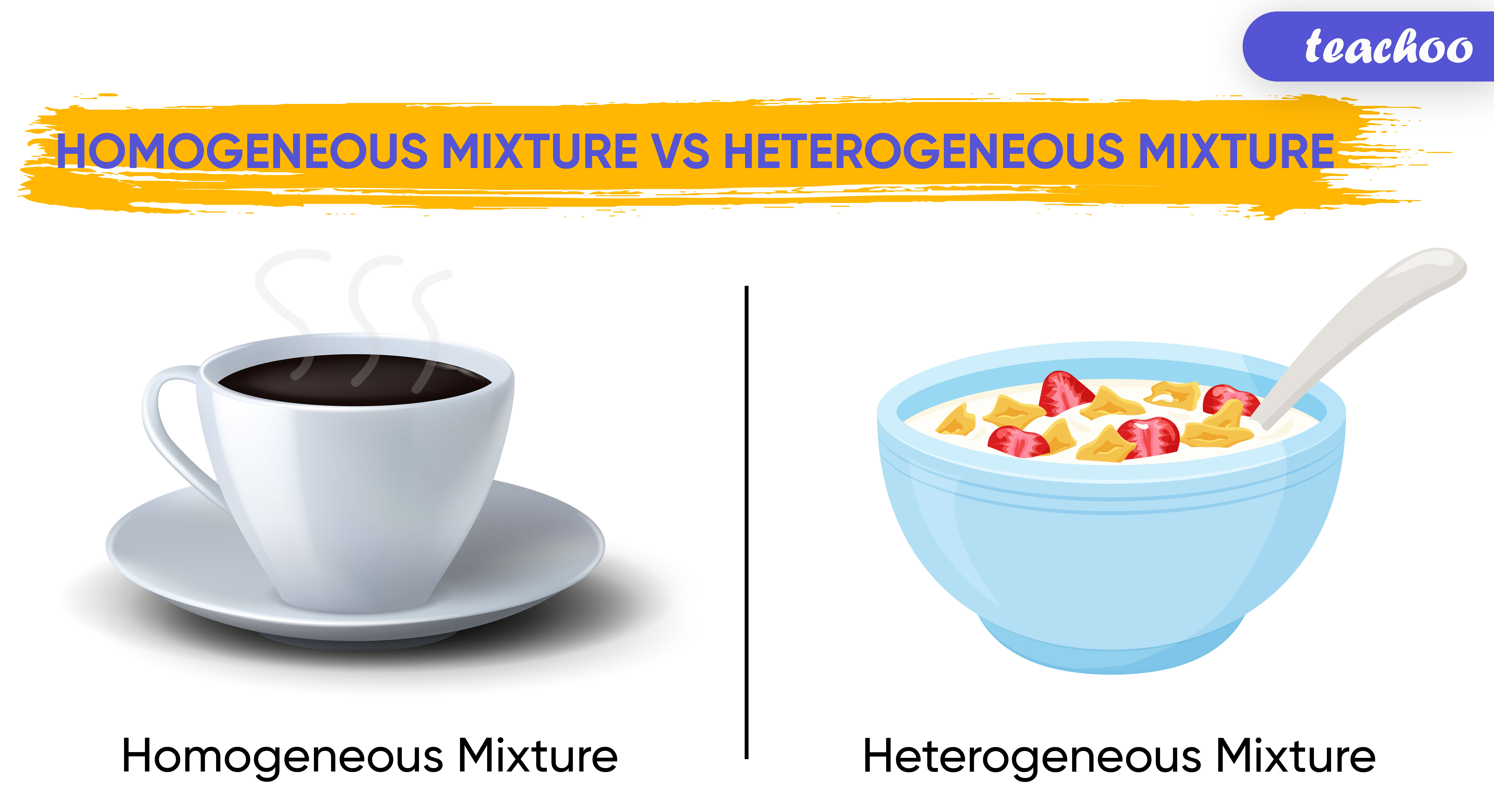
Heterogeneous vs. Homogeneous Mixtures
In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with its counterpart, homogeneous. The difference between heterogeneous and homogeneous mixtures is the degree to which the.
Homogeneous vs. Heterogeneous What's The Difference?
In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. Reactions that take place on the surface of a catalyst of a. A homogeneous mixture is a mixture in which the components. Heterogeneous and homogeneous systems possess distinct attributes that.
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo
Reactions that take place on the surface of a catalyst of a. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. A homogeneous mixture is a mixture in which the components. Heterogeneous and homogeneous systems possess distinct attributes that shape their behavior, interactions, and analysis. In this.
Reactions That Take Place On The Surface Of A Catalyst Of A.
A homogeneous mixture is a mixture in which the components. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. In this article, we’ll define homogeneous and heterogeneous, break down the differences between them, and provide some examples of the different things the words can describe, including both homogeneous and. Heterogeneous and homogeneous systems possess distinct attributes that shape their behavior, interactions, and analysis.
Homogeneous Reactions Are Chemical Reactions In Which The Reactants And Products Are In The Same Phase, While Heterogeneous Reactions Have Reactants In Two Or More Phases.
In imaging, a homogeneous appearance indicates uniformity. To grasp the concept of a heterogeneous appearance, it’s useful to contrast it with its counterpart, homogeneous. Heterogeneous systems are characterized by their diversity, lack of uniformity, potential for.




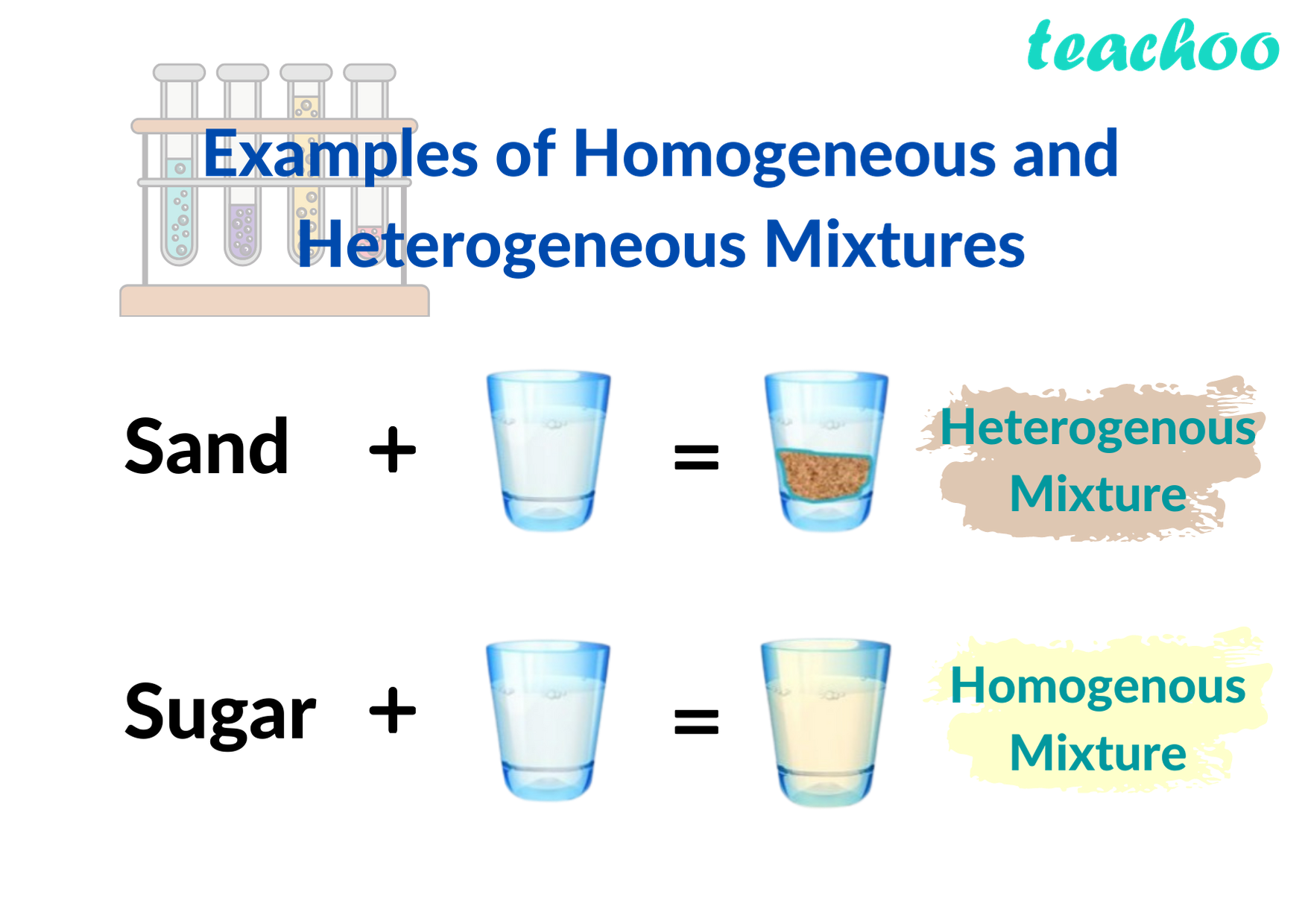

/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)