Chemistry Word Problems
Chemistry Word Problems - Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of sodium chloride in sea water? Write a balanced molecular equation describing each of the following chemical reactions.
Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of sodium chloride in sea water? Write a balanced molecular equation describing each of the following chemical reactions.
Sea water contains roughly 28.0 g of nacl per liter. Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water?
Dimensional Analysis Problems Worksheet Englishworksheet.my.id
Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water? Sea water contains roughly 28.0 g of nacl per liter.
Dimensional Analysis Worksheet Chemistry
Write a balanced molecular equation describing each of the following chemical reactions. Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of sodium chloride in sea water?
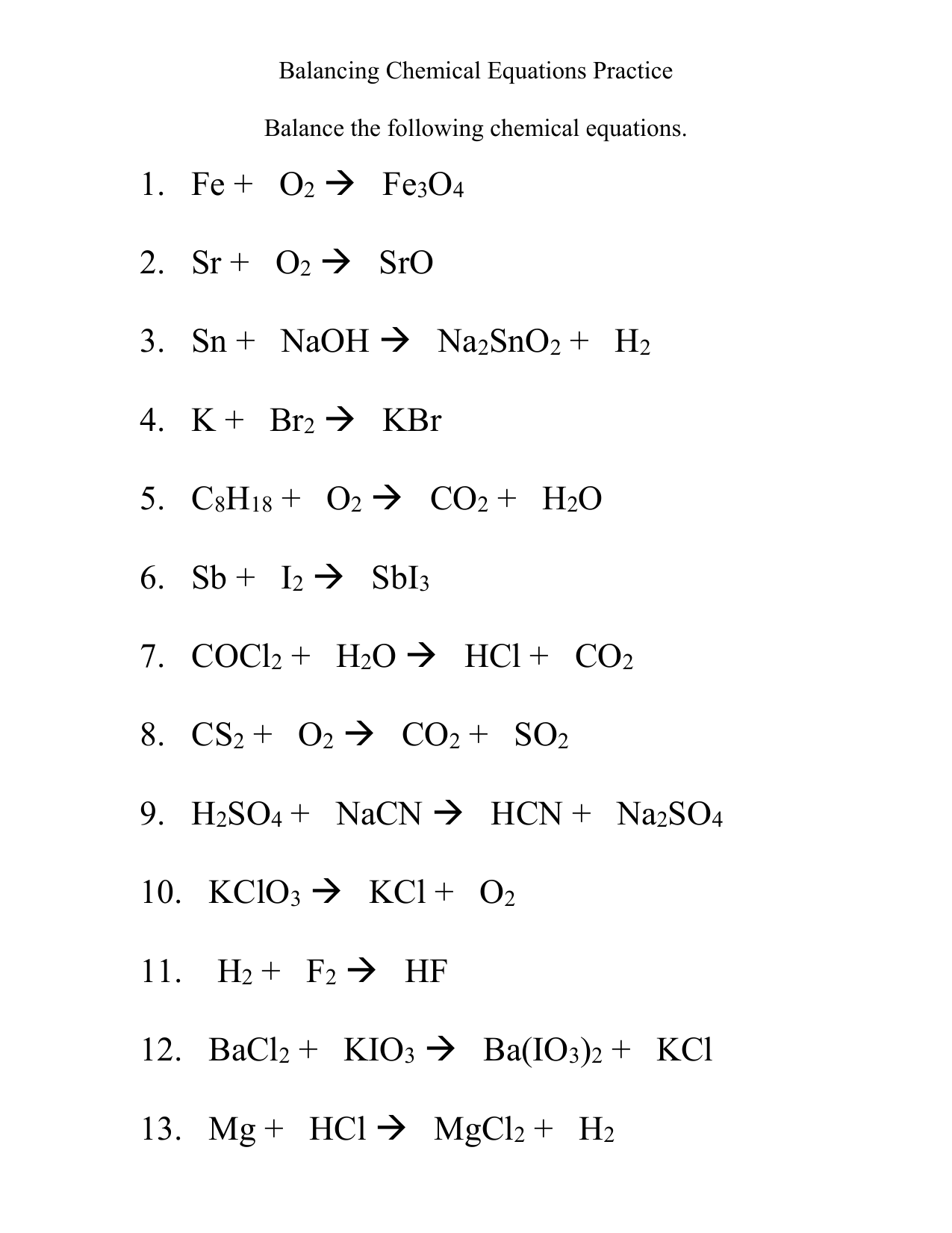
Balancing Chemical Equations Practice worksheet (1)
Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of sodium chloride in sea water? Write a balanced molecular equation describing each of the following chemical reactions.
Metric Conversion Worksheet Chemistry E Street Light
Sea water contains roughly 28.0 g of nacl per liter. Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water?
Chemistry Chemical Word Equations Worksheet Answers The Best Free
Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water? Sea water contains roughly 28.0 g of nacl per liter.
Chemistry Problems With Answers Science Notes and Projects
Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water? Sea water contains roughly 28.0 g of nacl per liter.
How to Solve Chemistry Word Problems
What is the molarity of sodium chloride in sea water? Write a balanced molecular equation describing each of the following chemical reactions. Sea water contains roughly 28.0 g of nacl per liter.
Worksheet Word Equations Equations Worksheets
Sea water contains roughly 28.0 g of nacl per liter. Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water?
Organic Chemistry Word Problem Question Chemistry Word Problems Class
Write a balanced molecular equation describing each of the following chemical reactions. What is the molarity of sodium chloride in sea water? Sea water contains roughly 28.0 g of nacl per liter.
What Is The Molarity Of Sodium Chloride In Sea Water?
Sea water contains roughly 28.0 g of nacl per liter. Write a balanced molecular equation describing each of the following chemical reactions.





:max_bytes(150000):strip_icc()/GettyImages-693801398-59b0389a054ad900107770d0.jpg)

