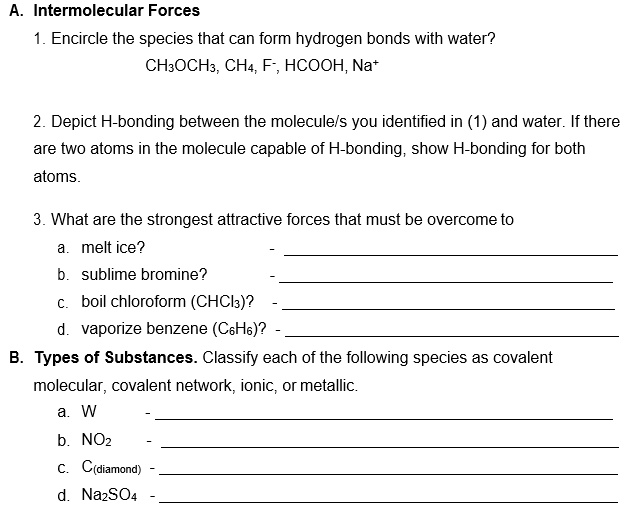
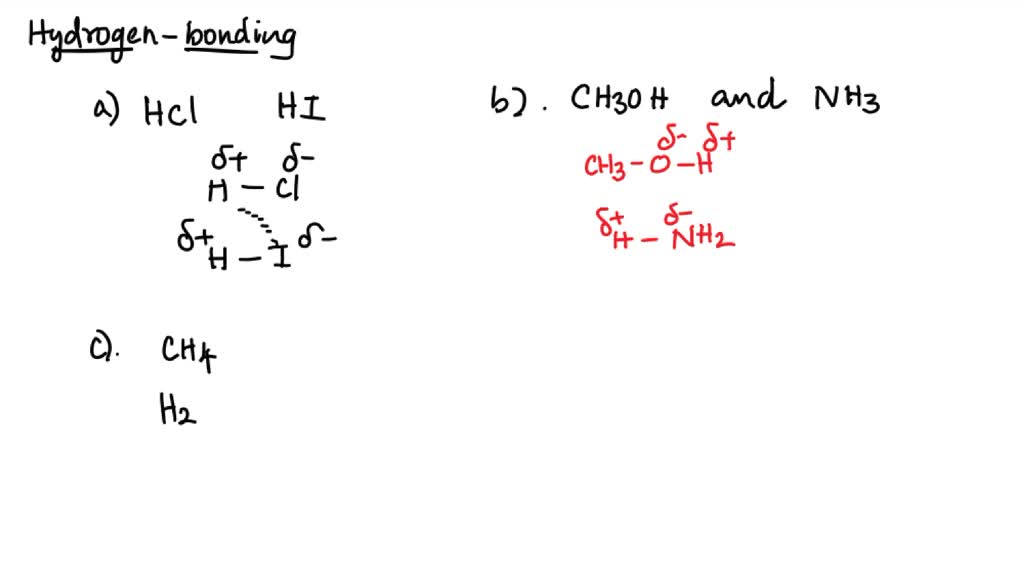
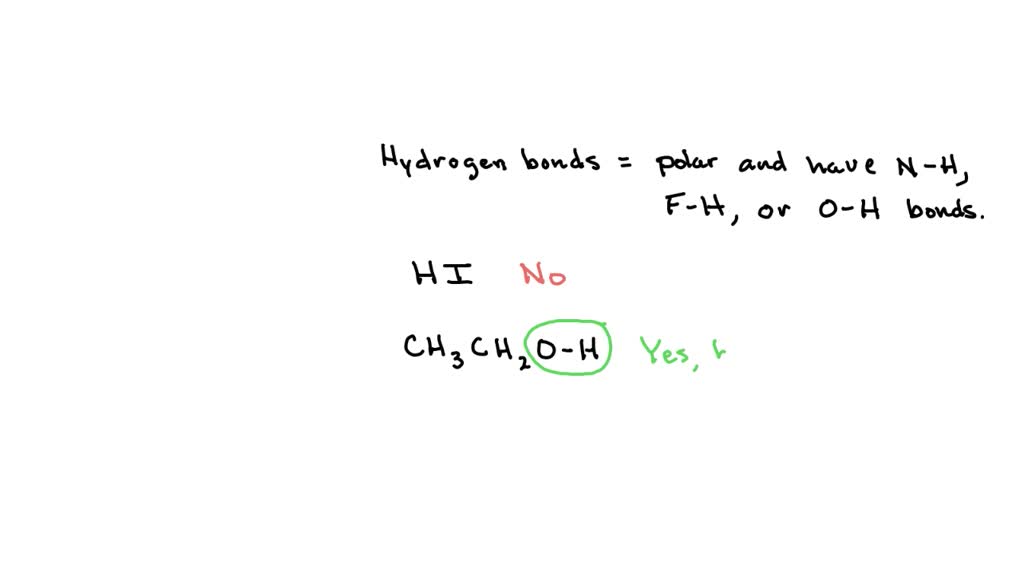
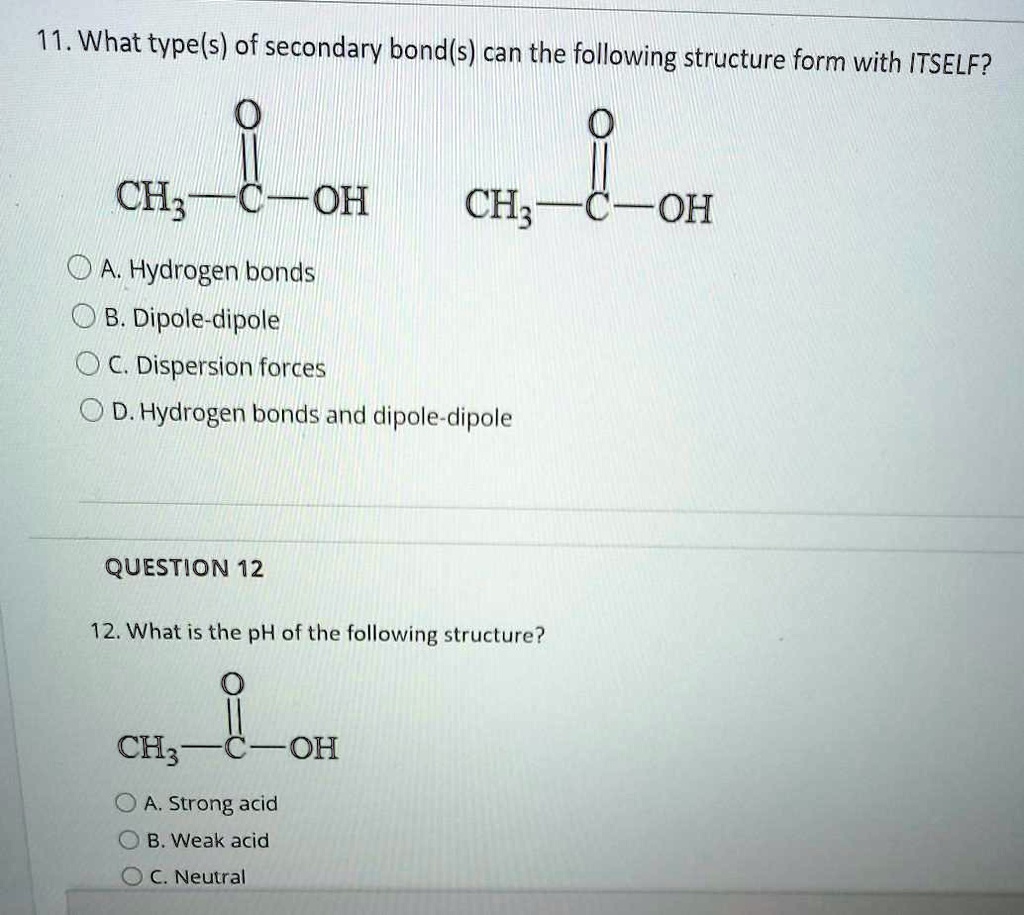
Can Ch3Oh Form Hydrogen Bonds
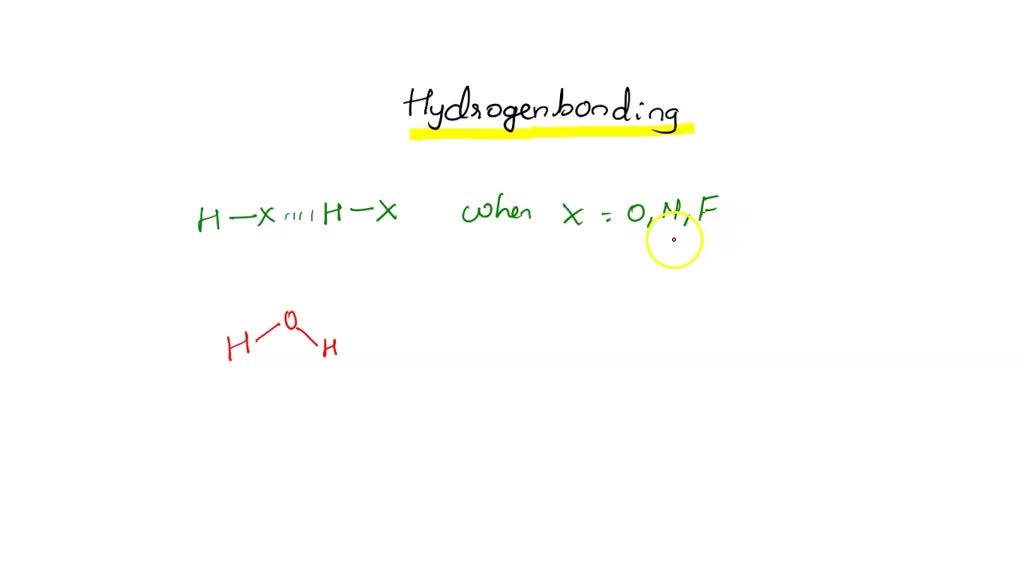
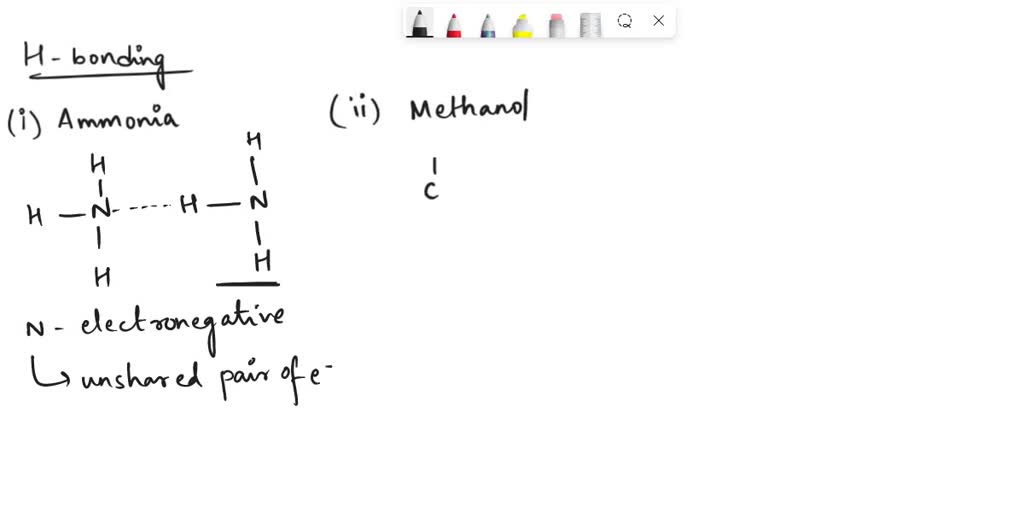
Can Ch3Oh Form Hydrogen Bonds - So, can ch3oh form hydrogen bonds? Ch₃br has no n, o, or f. It can form hydrogen bonds with other ch₃oh molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. In principle, the oxygen atom of ch3oh has two unused doublets. Each doublet can attract one h atom from foreign molecules. The answer is yes, but with some limitations. Ch3oh can form hydrogen bonds.
Ch3oh can form hydrogen bonds. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. It can form hydrogen bonds with other ch₃oh molecules. The answer is yes, but with some limitations. Ch₃br has no n, o, or f. In principle, the oxygen atom of ch3oh has two unused doublets. So, can ch3oh form hydrogen bonds? Each doublet can attract one h atom from foreign molecules.
Ch₃br has no n, o, or f. In principle, the oxygen atom of ch3oh has two unused doublets. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch3oh can form hydrogen bonds. It can form hydrogen bonds with other ch₃oh molecules. The answer is yes, but with some limitations. Each doublet can attract one h atom from foreign molecules. So, can ch3oh form hydrogen bonds?
SOLVED Intermolecular Forces Substances Intermolecular Forces 1
In principle, the oxygen atom of ch3oh has two unused doublets. So, can ch3oh form hydrogen bonds? It can form hydrogen bonds with other ch₃oh molecules. The answer is yes, but with some limitations. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.
Can Ch3oh Form Hydrogen Bonds Form example download
It can form hydrogen bonds with other ch₃oh molecules. So, can ch3oh form hydrogen bonds? Ch₃br has no n, o, or f. Each doublet can attract one h atom from foreign molecules. Ch3oh can form hydrogen bonds.
SOLVED 84 2 points Which pairs of compounds can hydrogen bond to each
In principle, the oxygen atom of ch3oh has two unused doublets. It can form hydrogen bonds with other ch₃oh molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch₃br has no n, o, or f. Each doublet can attract one h atom from foreign molecules.
SOLVED 11. What types of secondary bonds can the following structure
So, can ch3oh form hydrogen bonds? In principle, the oxygen atom of ch3oh has two unused doublets. Ch3oh can form hydrogen bonds. It can form hydrogen bonds with other ch₃oh molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.
SOLVED Which of the following can form hydrogen bonds? CHA CH3OH CH3F
In principle, the oxygen atom of ch3oh has two unused doublets. Ch3oh can form hydrogen bonds. Each doublet can attract one h atom from foreign molecules. It can form hydrogen bonds with other ch₃oh molecules. The answer is yes, but with some limitations.
SOLVED Which of the molecules can form a hydrogen bond with a water
So, can ch3oh form hydrogen bonds? Ch3oh can form hydrogen bonds. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch₃br has no n, o, or f. The answer is yes, but with some limitations.
Oxygen Covalent Bond Diagram
Each doublet can attract one h atom from foreign molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch3oh can form hydrogen bonds. In principle, the oxygen atom of ch3oh has two unused doublets. So, can ch3oh form hydrogen bonds?
SOLVED In which case will the two molecules be involved in dipole
It can form hydrogen bonds with other ch₃oh molecules. Each doublet can attract one h atom from foreign molecules. So, can ch3oh form hydrogen bonds? In principle, the oxygen atom of ch3oh has two unused doublets. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.
SOLVED a) Circle the molecules below that can form hydrogen bonds with
Ch3oh can form hydrogen bonds. In principle, the oxygen atom of ch3oh has two unused doublets. The answer is yes, but with some limitations. So, can ch3oh form hydrogen bonds? In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.
SOLVED Which of the following compounds can form hydrogen bonds with
In principle, the oxygen atom of ch3oh has two unused doublets. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch₃br has no n, o, or f. It can form hydrogen bonds with other ch₃oh molecules. Ch3oh can form hydrogen bonds.
Ch3Oh Can Form Hydrogen Bonds.
It can form hydrogen bonds with other ch₃oh molecules. In principle, the oxygen atom of ch3oh has two unused doublets. So, can ch3oh form hydrogen bonds? Each doublet can attract one h atom from foreign molecules.
In Summary, The Molecules That Can Form Hydrogen Bonds With Each Other Are Nh3, H2O, Ch3Oh, And Ch3Och3.
Ch₃br has no n, o, or f. The answer is yes, but with some limitations.